Revised Sublingual EV
Also updated titles in Nonbinary; Updated references in Genital E2; Updated track hash in multiple articlespull/11/head
parent
f7cb49e1e3
commit
ebc9c105ce
|
|
@ -12,7 +12,7 @@ tags:
|
|||
- 比卡鲁胺
|
||||
- 抗雄激素制剂
|
||||
- 用药安全
|
||||
trackHash: 9298d4aa52c9fdc517ff6c2e6231e528d3cdd1bc
|
||||
trackHash: 802de46c25d83106f6b875fc02d91ff2d69202bf
|
||||
keywords: [比卡鲁胺, 抗雄激素, 激素治疗, 安全性]
|
||||
---
|
||||
|
||||
|
|
|
|||
|
|
@ -14,7 +14,7 @@ tags:
|
|||
- 抗雄激素制剂
|
||||
- 用药安全
|
||||
- 用药途径与剂量
|
||||
trackHash: 88c1c3977dec49880f79a8d56d706505b9974a5c
|
||||
trackHash: 802de46c25d83106f6b875fc02d91ff2d69202bf
|
||||
keywords: [醋酸环丙孕酮, 色谱龙, 抗雄激素, 副作用, 用法用量]
|
||||
---
|
||||
|
||||
|
|
|
|||
|
|
@ -9,7 +9,7 @@ translated: 2023-03-28
|
|||
translators:
|
||||
- 昭心
|
||||
- Bersella AI
|
||||
trackHash: 9298d4aa52c9fdc517ff6c2e6231e528d3cdd1bc
|
||||
trackHash: 802de46c25d83106f6b875fc02d91ff2d69202bf
|
||||
tags:
|
||||
- 用药安全
|
||||
- 醋酸环丙孕酮
|
||||
|
|
|
|||
|
|
@ -12,7 +12,7 @@ translators:
|
|||
tags:
|
||||
- 雌激素
|
||||
- 用药途径与剂量
|
||||
trackHash: fdd701930bcb4e0f6f2c2ca8df31b80ae016e9a0
|
||||
trackHash: 43539e195ce7f91fa48a5c855392656dc179226d
|
||||
keywords: [雌二醇凝胶, 雌二醇贴片, 阴囊, 生殖器]
|
||||
---
|
||||
|
||||
|
|
@ -58,7 +58,7 @@ keywords: [雌二醇凝胶, 雌二醇贴片, 阴囊, 生殖器]
|
|||
Tratamiento del Cáncer de Próstata Avanzado con Estrógenos Transdérmicos Escrotales (ETE).
|
||||
\[阴囊给药的透皮雌激素贴片(TSEP)在晚期前列腺癌治疗当中的应用]
|
||||
_Revista Argentina de Urología, 70_(4), 231–241.
|
||||
[[Google 学术][P05]] [[网页地址][P05-ARCHIVE]] [[PDF 文档][P05-PDF]] [[英译文][P05-ENG]]
|
||||
\[[Google 学术][P05]] \[[网址][P05-ARCHIVE]] \[[PDF 文档][P05-PDF]] \[[英译文][P05-ENG]]
|
||||
|
||||
这应该是第一项、也是迄今唯一一项调研阴囊给药的透皮雌二醇的研究。其论文并非以英语撰写,因此早前的文献检索并未发现之。\
|
||||
在讨论该研究的结果之前,下文将会介绍关于阴囊给药的[透皮雌二醇][wiki18]的一些背景和原理。
|
||||
|
|
@ -215,28 +215,28 @@ Tegaderm 是一种透明薄膜敷料,可帮助固定贴片<sup>([Reddit 帖子
|
|||
|
||||
## 参考文献 {#references}
|
||||
|
||||
- Atkinson, L. E., Chang, Y. L., & Snyder, P. J. (1998). Long-term experience with testosterone replacement through scrotal skin. In Nieschlag, E., & Behre, H. M. (Eds.). _Testosterone: Action · Deficiency · Substitution, 2nd Edition_ (pp. 365–388). Berlin/Heidelberg: Springer. \[DOI:[10.1007/978-3-642-72185-4\_13](https://link.springer.com/chapter/10.1007/978-3-642-72185-4_13)\]
|
||||
- Behre, H. M., & Nieschlag, E. (2012). Testosterone preparations for clinical use in males. In Nieschlag, E., Behre, H. M., & Nieschlag, S. (Eds.). _Testosterone: Action · Deficiency · Substitution, 4th Edition_ (pp. 309–335). Cambridge/New York: Cambridge University Press. \[DOI:<https://doi.org/10.1017/CBO9781139003353.016>\]
|
||||
- Bensley, J. G., Cheung, A. S., Grossmann, M., & Papa, N. (2021). Testicular Cancer in Trans People Using Feminising Hormone Therapy– A Brief Review. _Urology_, advance online publication. \[DOI:<https://doi.org/10.1016/j.urology.2021.11.014>\]
|
||||
- de Nie, I., de Blok, C., van der Sluis, T. M., Barbé, E., Pigot, G., Wiepjes, C. M., Nota, N. M., van Mello, N. M., Valkenburg, N. E., Huirne, J., Gooren, L., van Moorselaar, R., Dreijerink, K., & den Heijer, M. (2020). Prostate Cancer Incidence under Androgen Deprivation: Nationwide Cohort Study in Trans Women Receiving Hormone Treatment. _The Journal of Clinical Endocrinology and Metabolism_, _105_(9), e3293–e3299. \[DOI:<https://doi.org/10.1210/clinem/dgaa412>\]
|
||||
- de Nie, I., Wiepjes, C. M., de Blok, C., van Moorselaar, R., Pigot, G., van der Sluis, T. M., Barbé, E., van der Voorn, P., van Mello, N. M., Huirne, J., & den Heijer, M. (2021). Incidence of testicular cancer in trans women using gender‐affirming hormonal treatment: a nationwide cohort study. _BJU International_, advance online publication. \[DOI:<https://doi.org/10.1111/bju.15575>\]
|
||||
- Feldmann, R. J., & Maibach, H. I. (1967). Regional variation in percutaneous penetration of 14C cortisol in man. _The Journal of Investigative Dermatology_, _48_(2), 181–183. \[DOI:<https://doi.org/10.1038/jid.1967.29>\]
|
||||
- Hairston, J. C., Becher, E., & McVary, K. T. (2006). Topical and Intra-Urethral Therapy. In Mulcahy, J. J. (Ed.). _Male Sexual Function: A Guide to Clinical Management, 2nd Edition_ (_Current Clinical Urology_) (pp. 303–321). Totowa: Humana Press. \[DOI:<https://doi.org/10.1007/978-1-59745-155-0_14>\] \[[Google 阅读](https://books.google.com/books?id=3ogNo5qAVJ8C&pg=PA309)\]
|
||||
- Iyer, R., Mok, S. F., Savkovic, S., Turner, L., Fraser, G., Desai, R., Jayadev, V., Conway, A. J., & Handelsman, D. J. (2017). Pharmacokinetics of testosterone cream applied to scrotal skin. _Andrology_, _5_(4), 725–731. \[DOI:<https://doi.org/10.1111/andr.12357>\]
|
||||
- Järvinen, A., Granander, M., Nykänen, S., Laine, T., Geurts, P., & Viitanen, A. (1997). Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. _British Journal of Obstetrics and Gynaecology_, _104_(Suppl 16), 14–18. \[DOI:<https://doi.org/10.1111/j.1471-0528.1997.tb11562.x>\]
|
||||
- Khera, M. (2013). Treatment Options for Testosterone Replacement Therapy. In Hellstrom, W. J. G. (Ed.). _Androgen Deficiency and Testosterone Replacement: Current Controversies and Strategies_ (pp. 129–139). Totowa: Humana Press. \[DOI:<https://doi.org/10.1007/978-1-62703-179-0_10>\]
|
||||
- Klein, K. O., Rosenfield, R. L., Santen, R. J., Gawlik, A. M., Backeljauw, P. F., Gravholt, C. H., Sas, T., & Mauras, N. (2018). Estrogen replacement in Turner syndrome: literature review and practical considerations. _The Journal of Clinical Endocrinology & Metabolism_, _103_(5), 1790–1803. \[DOI:<https://doi.org/10.1210/jc.2017-02183>\]
|
||||
- Kuhnert, B., Byrne, M., Simoni, M., Kopcke, W., Gerss, J., Lemmnitz, G., & Nieschlag, E. (2005). Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial. _European Journal of Endocrinology_, _153_(2), 317–326. \[DOI:<https://doi.org/10.1530/eje.1.01964>\]
|
||||
- Langley, R. E., Godsland, I. F., Kynaston, H., Clarke, N. W., Rosen, S. D., Morgan, R. C., Pollock, P., Kockelbergh, R., Lalani, e., Dearnaley, D., Parmar, M., & Abel, P. D. (2008). Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first‐line hormonal therapy in patients with locally advanced or metastatic prostate cancer. _BJU International_, _102_(4), 442–445. \[DOI:<https://doi.org/10.1111/j.1464-410X.2008.07583.x>\]
|
||||
- Langley, R. E., Gilbert, D. C., Duong, T., Clarke, N. W., Nankivell, M., Rosen, S. D., Mangar, S., Macnair, A., Sundaram, S. K., Laniado, M. E., Dixit, S., Madaan, S., Manetta, C., Pope, A., Scrase, C. D., Mckay, S., Muazzam, I. A., Collins, G. N., Worlding, J., Williams, S. T., … & Parmar, M. (2021). Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised Prostate Adenocarcinoma Transcutaneous Hormone (PATCH) trial programme. _The Lancet_, _397_(10274), 581–591. \[DOI:<https://doi.org/10.1016/S0140-6736(21)00100-8>\]
|
||||
- Matthews, D., Bath, L., Högler, W., Mason, A., Smyth, A., & Skae, M. (2017). Hormone supplementation for pubertal induction in girls. _Archives of Disease in Childhood_, _102_(10), 975–980. \[DOI:[10.1136/archdischild-2016-311372](http://dx.doi.org/10.1136/archdischild-2016-311372)\]
|
||||
- Mazer, N. A., Heiber, W. E., Moellmer, J. F., Meikle, A. W., Stringham, J. D., Sanders, S. W., Tolman, K. G., & Odell, W. D. (1992). Enhanced transdermal delivery of testosterone: a new physiological approach for androgen replacement in hypogonadal men. _Journal of Controlled Release_, _19_(1–3), 347–361. \[DOI:<https://doi.org/10.1016/0168-3659(92)90089-A>\]
|
||||
- Norjavaara, E., Ankarberg-Lindgren, C., & Kriström, B. (2016). Sex steroid replacement therapy in female hypogonadism from childhood to young adulthood. In Bourguignon J.-P., Parent A.-S. (Eds.). _Puberty from Bench to Clinic. Lessons for Clinical Management of Pubertal Disorders_ (_Endocrine Development, Volume_ _29_) (pp. 198–213). Basel: Karger. \[DOI:<https://doi.org/10.1159/000438892>\]
|
||||
- Norman, G., Dean, M. E., Langley, R. E., Hodges, Z. C., Ritchie, G., Parmar, M. K., Sydes, M. R., Abel, P., & Eastwood, A. J. (2008). Parenteral oestrogen in the treatment of prostate cancer: a systematic review. _British Journal of Cancer_, _98_(4), 697–707. \[DOI:<https://doi.org/10.1038/sj.bjc.6604230>\]
|
||||
- Ockrim, J., Lalani, E. N., & Abel, P. (2006). Therapy insight: parenteral estrogen treatment for prostate cancer—a new dawn for an old therapy. _Nature clinical practice Oncology_, _3_(10), 552–563. \[DOI:<https://doi.org/10.1038/ncponc0602>\]
|
||||
- Place, V. A., Atkinson, L., Prather, D. A., Trunnell, N., & Yates, F. E. (1990). Transdermal testosterone replacement through genital skin. In Nieschlag, E., & Behre, H. M. (Eds.). _Testosterone: Action · Deficiency · Substitution, 1st Edition_ (pp. 165–181). Berlin/Heidelberg: Springer. \[DOI:<https://doi.org/10.1007/978-3-662-00814-0_9>\]
|
||||
- Premoli, F., Re, I., Asenjo, G., Maximino, G., & Micheletti, L. (2005). Tratamiento del Cáncer de Próstata Avanzado con Estrógenos Transdérmicos Escrotales (ETE). \[Transdermal Scrotal Estrogen Patches (TSEP) in the Treatment of Advanced Prostate Cancer.\] _Revista Argentina de Urología_, _70_(4), 231–241. \[[Google 学术](https://scholar.google.com/scholar?cluster=3203160436559309066)\] \[[URL](https://web.archive.org/web/20201112031803/https://www.sau-net.org/publicaciones/abstracts_70_4.html)\] \[[PDF](https://revistasau.org/index.php/revista/article/viewFile/3224/3168)\] \[[英译文](https://docs.google.com/document/d/1dteNhG9wewvNX5zj0YcXrVAuQFZdtcqWmUWVvgI5uKs/view)\]
|
||||
- Rosenfield, R. L., Kiess, W., & de Muinck Keizer-Schrama, S. (2006). Physiologic induction of puberty in Turner syndrome with very low-dose estradiol. _International Congress Series_, _1298_, 71–79. \[DOI:<https://doi.org/10.1016/j.ics.2006.07.003>\]
|
||||
- Atkinson, L. E., Chang, Y. L., & Snyder, P. J. (1998). Long-term experience with testosterone replacement through scrotal skin. In Nieschlag, E., & Behre, H. M. (Eds.). *Testosterone: Action · Deficiency · Substitution, 2nd Edition* (pp. 365–388). Berlin/Heidelberg: Springer. \[DOI:[10.1007/978-3-642-72185-4\_13][ACS98]] \[[备用][ACS98-ALT]]
|
||||
- Behre, H. M., & Nieschlag, E. (2012). Testosterone preparations for clinical use in males. In Nieschlag, E., Behre, H. M., & Nieschlag, S. (Eds.). *Testosterone: Action · Deficiency · Substitution, 4th Edition* (pp. 309–335). Cambridge/New York: Cambridge University Press. \[DOI:[10.1017/CBO9781139003353.016][BN12-DOI]] \[[Google 阅读][bn12]]
|
||||
- Bensley, J. G., Cheung, A. S., Grossmann, M., & Papa, N. (2022). Testicular Cancer in Trans People Using Feminising Hormone Therapy– A Brief Review. *Urology*, *160*, 1–4. \[DOI:[10.1016/j.urology.2021.11.014][B21]]
|
||||
- de Nie, I., de Blok, C., van der Sluis, T. M., Barbé, E., Pigot, G., Wiepjes, C. M., Nota, N. M., van Mello, N. M., Valkenburg, N. E., Huirne, J., Gooren, L., van Moorselaar, R., Dreijerink, K., & den Heijer, M. (2020). Prostate Cancer Incidence under Androgen Deprivation: Nationwide Cohort Study in Trans Women Receiving Hormone Treatment. *The Journal of Clinical Endocrinology and Metabolism*, *105*(9), e3293–e3299. \[DOI:[10.1210/clinem/dgaa412][N20]]
|
||||
- de Nie, I., Wiepjes, C. M., Blok, C. J., Moorselaar, R. J., Pigot, G. L., van der Sluis, T. M., Barbé, E., van der Voorn, P., van Mello, N. M., Huirne, J., & den Heijer, M. (2021). Incidence of testicular cancer in trans women using gender‐affirming hormonal treatment: a nationwide cohort study. *BJU International*, *129*(4), 491–497. \[DOI:[10.1111/bju.15575][N21]]
|
||||
- Feldmann, R. J., & Maibach, H. I. (1967). Regional variation in percutaneous penetration of 14C cortisol in man. *The Journal of Investigative Dermatology*, *48*(2), 181–183. \[DOI:[10.1038/jid.1967.29][FM67]]
|
||||
- Hairston, J. C., Becher, E., & McVary, K. T. (2006). Topical and Intra-Urethral Therapy. In Mulcahy, J. J. (Ed.). *Male Sexual Function: A Guide to Clinical Management, 2nd Edition* (*Current Clinical Urology*) (pp. 303–321). Totowa: Humana Press. \[DOI:[10.1007/978-1-59745-155-0\_14][HBM06]] \[[Google 阅读][HBM06-GB]]
|
||||
- Iyer, R., Mok, S. F., Savkovic, S., Turner, L., Fraser, G., Desai, R., Jayadev, V., Conway, A. J., & Handelsman, D. J. (2017). Pharmacokinetics of testosterone cream applied to scrotal skin. *Andrology*, *5*(4), 725–731. \[DOI:[10.1111/andr.12357][I17]]
|
||||
- Järvinen, A., Granander, M., Nykänen, S., Laine, T., Geurts, P., & Viitanen, A. (1997). Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. *British Journal of Obstetrics and Gynaecology*, *104*(Suppl 16), 14–18. \[DOI:[10.1111/j.1471-0528.1997.tb11562.x][J97]]
|
||||
- Khera, M. (2013). Treatment Options for Testosterone Replacement Therapy. In Hellstrom, W. J. G. (Ed.). *Androgen Deficiency and Testosterone Replacement: Current Controversies and Strategies* (pp. 129–139). Totowa: Humana Press. \[DOI:[10.1007/978-1-62703-179-0\_10][K13]]
|
||||
- Klein, K. O., Rosenfield, R. L., Santen, R. J., Gawlik, A. M., Backeljauw, P. F., Gravholt, C. H., Sas, T., & Mauras, N. (2018). Estrogen replacement in Turner syndrome: literature review and practical considerations. *The Journal of Clinical Endocrinology & Metabolism*, *103*(5), 1790–1803. \[DOI:[10.1210/jc.2017-02183][K18]]
|
||||
- Kuhnert, B., Byrne, M., Simoni, M., Kopcke, W., Gerss, J., Lemmnitz, G., & Nieschlag, E. (2005). Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial. *European Journal of Endocrinology*, *153*(2), 317–326. \[DOI:[10.1530/eje.1.01964][K05]]
|
||||
- Langley, R. E., Godsland, I. F., Kynaston, H., Clarke, N. W., Rosen, S. D., Morgan, R. C., Pollock, P., Kockelbergh, R., Lalani, e., Dearnaley, D., Parmar, M., & Abel, P. D. (2008). Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first‐line hormonal therapy in patients with locally advanced or metastatic prostate cancer. *BJU International*, *102*(4), 442–445. \[DOI:[10.1111/j.1464-410X.2008.07583.x][L08]]
|
||||
- Langley, R. E., Gilbert, D. C., Duong, T., Clarke, N. W., Nankivell, M., Rosen, S. D., Mangar, S., Macnair, A., Sundaram, S. K., Laniado, M. E., Dixit, S., Madaan, S., Manetta, C., Pope, A., Scrase, C. D., Mckay, S., Muazzam, I. A., Collins, G. N., Worlding, J., Williams, S. T., … & Parmar, M. (2021). Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised Prostate Adenocarcinoma Transcutaneous Hormone (PATCH) trial programme. *The Lancet*, *397*(10274), 581–591. \[DOI:[10.1016/S0140-6736(21)00100-8][L21]]
|
||||
- Matthews, D., Bath, L., Högler, W., Mason, A., Smyth, A., & Skae, M. (2017). Hormone supplementation for pubertal induction in girls. *Archives of Disease in Childhood*, *102*(10), 975–980. \[DOI:[10.1136/archdischild-2016-311372][M17]]
|
||||
- Mazer, N. A., Heiber, W. E., Moellmer, J. F., Meikle, A. W., Stringham, J. D., Sanders, S. W., Tolman, K. G., & Odell, W. D. (1992). Enhanced transdermal delivery of testosterone: a new physiological approach for androgen replacement in hypogonadal men. *Journal of Controlled Release*, *19*(1–3), 347–361. \[DOI:[10.1016/0168-3659(92)90089-A][M92]]
|
||||
- Norjavaara, E., Ankarberg-Lindgren, C., & Kriström, B. (2016). Sex steroid replacement therapy in female hypogonadism from childhood to young adulthood. In Bourguignon J.-P., Parent A.-S. (Eds.). *Puberty from Bench to Clinic. Lessons for Clinical Management of Pubertal Disorders* (*Endocrine Development, Volume* *29*) (pp. 198–213). Basel: Karger. \[DOI:[10.1159/000438892][NAK16]]
|
||||
- Norman, G., Dean, M. E., Langley, R. E., Hodges, Z. C., Ritchie, G., Parmar, M. K., Sydes, M. R., Abel, P., & Eastwood, A. J. (2008). Parenteral oestrogen in the treatment of prostate cancer: a systematic review. *British Journal of Cancer*, *98*(4), 697–707. \[DOI:[10.1038/sj.bjc.6604230][N08]]
|
||||
- Ockrim, J., Lalani, E. N., & Abel, P. (2006). Therapy insight: parenteral estrogen treatment for prostate cancer—a new dawn for an old therapy. *Nature clinical practice Oncology*, *3*(10), 552–563. \[DOI:[10.1038/ncponc0602][OLA06]]
|
||||
- Place, V. A., Atkinson, L., Prather, D. A., Trunnell, N., & Yates, F. E. (1990). Transdermal testosterone replacement through genital skin. In Nieschlag, E., & Behre, H. M. (Eds.). *Testosterone: Action · Deficiency · Substitution, 1st Edition* (pp. 165–181). Berlin/Heidelberg: Springer. \[DOI:[10.1007/978-3-662-00814-0\_9][P90]]
|
||||
- Premoli, F., Re, I., Asenjo, G., Maximino, G., & Micheletti, L. (2005). Tratamiento del Cáncer de Próstata Avanzado con Estrógenos Transdérmicos Escrotales (ETE). \[Transdermal Scrotal Estrogen Patches (TSEP) in the Treatment of Advanced Prostate Cancer.] *Revista Argentina de Urología*, *70*(4), 231–241. \[[Google 学术][P05]] \[[URL][P05-ARCHIVE]] \[[PDF][P05-PDF]] \[[英译本][P05-ENG]]
|
||||
- Rosenfield, R. L., Kiess, W., & de Muinck Keizer-Schrama, S. (2006). Physiologic induction of puberty in Turner syndrome with very low-dose estradiol. *International Congress Series*, *1298*, 71–79. \[DOI:[10.1016/j.ics.2006.07.003][RKK06]]
|
||||
|
||||
---
|
||||
|
||||
|
|
@ -246,6 +246,7 @@ Tegaderm 是一种透明薄膜敷料,可帮助固定贴片<sup>([Reddit 帖子
|
|||
时间,备注
|
||||
2021 年 10 月 23 日,首次翻译。
|
||||
2022 年 11 月 14 日,第一次修订,梳理全文叙述,增补“用药安全性”一节,整理外链。
|
||||
2023 年 3 月 30 日,更新“参考文献”和部分链接。
|
||||
```
|
||||
|
||||
<!-- 图表外链 -->
|
||||
|
|
@ -260,7 +261,7 @@ Tegaderm 是一种透明薄膜敷料,可帮助固定贴片<sup>([Reddit 帖子
|
|||
|
||||
<!-- 表格外链 -->
|
||||
|
||||
[table1]: https://en.wikipedia.org/wiki/Template:Transdermal_estradiol_patches_marketed_in_the_United_States
|
||||
[table1]: https://en.wikipedia.org/w/index.php?title=Template:Transdermal_estradiol_patches_marketed_in_the_United_States&oldid=962671786
|
||||
|
||||
<!-- 维基百科条目 -->
|
||||
|
||||
|
|
@ -342,7 +343,7 @@ Tegaderm 是一种透明薄膜敷料,可帮助固定贴片<sup>([Reddit 帖子
|
|||
[p05]: https://scholar.google.com/scholar?cluster=3203160436559309066
|
||||
[p05-archive]: https://web.archive.org/web/20201112031803/https://www.sau-net.org/publicaciones/abstracts_70_4.html
|
||||
[p05-pdf]: https://revistasau.org/index.php/revista/article/viewFile/3224/3168
|
||||
[p05-eng]: https://docs.google.com/document/d/1dteNhG9wewvNX5zj0YcXrVAuQFZdtcqWmUWVvgI5uKs/view
|
||||
[p05-eng]: https://files.transfemscience.org/pdfs/translations/Premoli%20et%20al.%20(2005)%20-%20Tratamiento%20del%20C%C3%A1ncer%20de%20Pr%C3%B3stata%20Avanzado%20con%20Estr%C3%B3genos%20Transd%C3%A9rmicos%20Escrotales%20%5BTransdermal%20Scrotal%20Estrogen%20Patches%20in%20the%20Treatment%20of%20Advanced%20Prostate%20Cancer%5D.pdf
|
||||
[aw20-ecbc]: https://transfemscience.org/articles/estrogens-coagulation-blood-clots/
|
||||
[ola06]: https://doi.org/10.1038/ncponc0602
|
||||
[l08]: https://doi.org/10.1111/j.1464-410X.2008.07583.x
|
||||
|
|
@ -364,3 +365,9 @@ Tegaderm 是一种透明薄膜敷料,可帮助固定贴片<sup>([Reddit 帖子
|
|||
[n20]: https://doi.org/10.1210/clinem/dgaa412
|
||||
[n08]: https://doi.org/10.1038/sj.bjc.6604230
|
||||
[l21]: https://doi.org/10.1016/S0140-6736(21)00100-8
|
||||
|
||||
[ACS98-ALT]: https://link.springer.com/chapter/10.1007/978-3-642-72185-4_13
|
||||
[BN12-DOI]: https://doi.org/10.1017/CBO9781139003353.016
|
||||
[HBM06-GB]: https://books.google.com/books?id=3ogNo5qAVJ8C&pg=PA309
|
||||
[M17]: https://dx.doi.org/10.1136/archdischild-2016-311372
|
||||
|
||||
|
|
|
|||
|
|
@ -15,7 +15,7 @@ tags:
|
|||
- SERM
|
||||
- 抗雄激素制剂
|
||||
notice: 原文 [最初以 Reddit 帖子的形式发布]({{< ref "announcement" >}}),在迁移至本站之后,其尚未得到适当或全面的修订。
|
||||
trackHash: fdd701930bcb4e0f6f2c2ca8df31b80ae016e9a0
|
||||
trackHash: 43539e195ce7f91fa48a5c855392656dc179226d
|
||||
keywords: [HRT, 非二元, 激素治疗, non-binary, SERM, MtX]
|
||||
---
|
||||
|
||||
|
|
@ -258,7 +258,7 @@ SERM 可有效维持骨密度。不过很遗憾,SERM 在骨骼内仅表达部
|
|||
|
||||
## 后记 {#updates}
|
||||
|
||||
### 后记一 {#update-1}
|
||||
### 后记一:Xu 等人 (2021) 所作评述 {#update-1-xu-et-al-2021}
|
||||
|
||||
2021 年六月,一篇关于 SERM 用于非二元(女性倾向)跨性别者的评述公开发表:
|
||||
|
||||
|
|
@ -273,17 +273,17 @@ Ada S. Cheung 是该论文的联名作者之一;其来自澳大利亚墨尔本
|
|||
|
||||
在此前文献中,还出现了少量关于 SERM 用于非二元(女性倾向与男性倾向)跨性别者的叙述;不过其提供的资料更为有限<sup>([Carswell & Roberts, 2017][cr17]; [Moser & Devereux, 2019][md19]; [Pang et al., 2020][pang20]; [Naroji et al., 2021][n21])</sup>。
|
||||
|
||||
### 后记二 {#update-2}
|
||||
### 后记二:van Dijken 等人 (2022) 和 Cocchetti 等人 (2022) 所作论文 {#update-2-van-dijken-et-al-2022-and-cocchetti-et-al-2022}
|
||||
|
||||
在最早的有关为非二元性别人群定制的激素疗法的研究当中,有一项于 2022 年三月公开发表了文献:
|
||||
|
||||
- van Dijken, J. B., Steensma, T. D., Wensing-Kruger, S. A., Heijer, M. D., & Dreijerink, K. M. (2022).
|
||||
**Tailored Gender-Affirming Hormone Treatment in Nonbinary Transgender Individuals: A Retrospective Study in a Referral Center Cohort.** [某治疗转诊中心的一项回顾性研究:为非二元跨性别者定制的性别肯定激素治疗]
|
||||
*Transgender Health*, 在线发表 (ahead of print). [DOI:[10.1089/trgh.2021.0032][d22]]
|
||||
*Transgender Health*, 先行在线发表 (advance online publication). \[DOI:[10.1089/trgh.2021.0032][d22]]
|
||||
|
||||
此外,有一篇关于[欧洲性别不一致调查组织网络][wiki76](ENIGI)所做工作的评述,还描述了 ENIGI 在将来针对非二元性别人群之激素治疗的研究计划:
|
||||
|
||||
- Cocchetti, C., Romani, A., Collet, S., Greenman, Y., Schreiner, T., Wiepjes, C., … & Fisher, A. D. (2022).
|
||||
- Cocchetti, C., Romani, A., Collet, S., Greenman, Y., Schreiner, T., Wiepjes, C., den Heijer, M., T’Sjoen, G., & Fisher, A. D. (2022).
|
||||
**The ENIGI (European Network for the Investigation of Gender Incongruence) Study: Overview of Acquired Endocrine Knowledge and Future Perspectives.** [对 ENIGI 项目已有的内分泌学认识与未来焦点的综述]
|
||||
*Journal of Clinical Medicine, 11*(7), 1784. [DOI:[10.3390/jcm11071784][c22]]
|
||||
|
||||
|
|
@ -311,6 +311,7 @@ Ada S. Cheung 是该论文的联名作者之一;其来自澳大利亚墨尔本
|
|||
时间,备注
|
||||
2021 年 8 月 6 日,首次翻译。
|
||||
2022 年 11 月 22 日,第一次修订,优化全文叙述,增补“后记二”内容,跟进移除“结论”一章。
|
||||
2023 年 3 月 30 日,更新“后记”标题。
|
||||
```
|
||||
|
||||
<!-- 维基百科/词典条目 -->
|
||||
|
|
|
|||
|
|
@ -11,7 +11,7 @@ tags:
|
|||
- 孕激素
|
||||
- 孕酮
|
||||
- 用药途径与剂量
|
||||
trackHash: fdd701930bcb4e0f6f2c2ca8df31b80ae016e9a0
|
||||
trackHash: 43539e195ce7f91fa48a5c855392656dc179226d
|
||||
keywords: [孕激素, 孕酮, 黄体酮, 空孕, 副作用, 用法, 用量]
|
||||
---
|
||||
|
||||
|
|
|
|||
|
|
@ -11,7 +11,7 @@ translators:
|
|||
tags:
|
||||
- 雌激素
|
||||
- 用药途径与剂量
|
||||
trackHash: fdd701930bcb4e0f6f2c2ca8df31b80ae016e9a0
|
||||
trackHash: 43539e195ce7f91fa48a5c855392656dc179226d
|
||||
keywords: [补佳乐, 诺坤复, 雌二醇, 舌下含服, 用法用量]
|
||||
---
|
||||
|
||||
|
|
|
|||
|
|
@ -1,112 +0,0 @@
|
|||
---
|
||||
title: 关于女性倾向跨性别者舌下含服戊酸雌二醇片剂的简介
|
||||
linkTitle: 舌下含服戊酸雌二醇的可行性
|
||||
description: 戊酸雌二醇的舌下含服途径尽管鲜有研究,但其与含服雌二醇同样有效,也可作为口服途径的替代。
|
||||
author: Aly
|
||||
published: 2019-01-05
|
||||
updated: 2021-09-07
|
||||
translated: 2022-02-18
|
||||
translators:
|
||||
- Bersella AI
|
||||
tags:
|
||||
- 雌激素
|
||||
- 用药途径与剂量
|
||||
trackHash: fdd701930bcb4e0f6f2c2ca8df31b80ae016e9a0
|
||||
keywords: [Progynova, 补佳乐, 泰补, 戊酸雌二醇片, 含服, 舌下含服, 用法]
|
||||
---
|
||||
|
||||
## 免责声明 {#notice}
|
||||
|
||||
**本文不构成任何医疗、处方建议。** 如有医疗需要,应于专业医师指导下进行。
|
||||
因译者能力所限,部分术语之翻译或有纰漏,烦请指正。
|
||||
|
||||
---
|
||||
|
||||
## 摘要 {#abstract}
|
||||
|
||||
口服用雌二醇片可经舌下含服,此举相较口服可达到更高的生物利用度,以及雌二醇水平。因此,舌下含服雌二醇在女性倾向跨性别者当中被广泛采用。在世界部分地区也有口服用的戊酸雌二醇(EV)片,常有女性倾向跨性别者咨询其被舌下含服时是否也能高效利用。
|
||||
|
||||
这方面的研究很有限,尚无二者的直接对比;不过至少,已有一项在顺性别女性当中进行的研究表明,EV 片舌下含服时可引起较高的雌二醇水平,以及和雌二醇片类似的对睾酮之抑制。此外,已有至少一座性别肯定激素治疗(GAHT)诊所报告了女性倾向跨性别者使用舌下含服的 EV。因此,舌下含服 EV 似乎是跟舌下含服雌二醇相似的一种高效给药途径。
|
||||
|
||||
二者之间有一处不同:EV 的雌二醇含量更少,故所需摄入量也略多。此外,雌二醇片与 EV 片并非为舌下含服而准备与设计(尽管很有效),其剂型亦有不同。在临床特性上,*(不同剂型)*可能的影响尚未可知。不过显然,不同剂型的片剂舌下含服所需吸收时间会有明显差别。这些剂型的一些性状,例如微粉化、亲油性等,对其药代动力学的影响也会有异,而这尚未得到研究。
|
||||
|
||||
那么,选择舌下含服雌二醇,而非 EV,应是明智之举,因为有关前者的描述更丰富,不确定性更少。不过显然,经舌下含服的 EV 对于女性倾向跨性别者也会很有效。
|
||||
|
||||
## 介绍 {#introduction}
|
||||
|
||||
雌二醇片[如诺坤复(Estrofem)、Estrace 等]口服后,通常会被吞服。然而,作为通常的吞服途径之替代,雌二醇片也被用于舌下含服,或含于面颊或嘴唇、牙龈处。舌下、面颊含服雌二醇片相较口服,可达到更高的生物利用度,以及雌二醇水平 ([Sam, 2021](https://transfemscience.org/articles/sublingual-e2-transfem/))。
|
||||
|
||||
女性倾向跨性别者在激素治疗当中,常采用舌下含服雌二醇。在一些国家(例如在欧洲),口服用雌二醇会以戊酸雌二醇[EV,如补佳乐(Progynova)]片的形式提供。经常有女性倾向跨性别者咨询,**EV 片是否也能像雌二醇片那样被舌下含服,以及二者是否会有差异**。
|
||||
|
||||
本文旨在探讨并聚焦于这些问题。
|
||||
|
||||
## EV 舌下含服之有效性 {#effectiveness-of-sublingual-estradiol-valerate}
|
||||
|
||||
EV 是一种雌二醇酯,是雌二醇的前体。雌二醇酯本身在被转换成雌二醇前,并不具备药理活性。EV 及其它雌二醇酯,会被各种酯酶裂解为雌二醇。这些酯酶遍布全身;从雌二醇酯到雌二醇的代谢过程,除肝脏外,也见于血液和其它组织中 ([维基百科](https://en.wikipedia.org/wiki/Estradiol_valerate#Pharmacokinetics))。因此,形如 EV 的雌二醇酯,可以不经过肝脏的首过效应(口服后转换为雌二醇时会发生)。综上,对于非口服方式(如舌下含服,以及肌注)的 EV,其之转换不会成为一种障碍。
|
||||
|
||||
关于舌下含服(而非口服)雌二醇片的研究很有限,而针对 EV 片的研究则严重缺失。至今尚无在舌下含服雌二醇片与 EV 片之间进行的直接比较。因此,关于舌下含服 EV 在药代动力学上(例如生物利用度、雌二醇水平、雌二醇浓度—时间曲线)相较雌二醇片表现如何,我们暂无可靠数据。
|
||||
|
||||
只有一项研究调查并描述了 EV 片的舌下含服。这项研究对一组每日以口服用 EV 片(“补佳乐”品牌)进行 3\~4 次舌下含服的,绝经前的顺性别妇女进行了评估。研究发现在以下两篇论文被发表:
|
||||
|
||||
- Serhal, P., & Craft, I. (1989). Oocyte donation in 61 patients. The Lancet, 333(8648), 1185–1187. \[DOI: <https://doi.org/10.1016/S0140-6736(89)92762-1>]
|
||||
- Serhal, P. (1990). Oocyte donation and surrogacy. British Medical Bulletin, 46(3), 796–812. \[DOI: <https://doi.org/10.1093/oxfordjournals.bmb.a072432>]
|
||||
|
||||
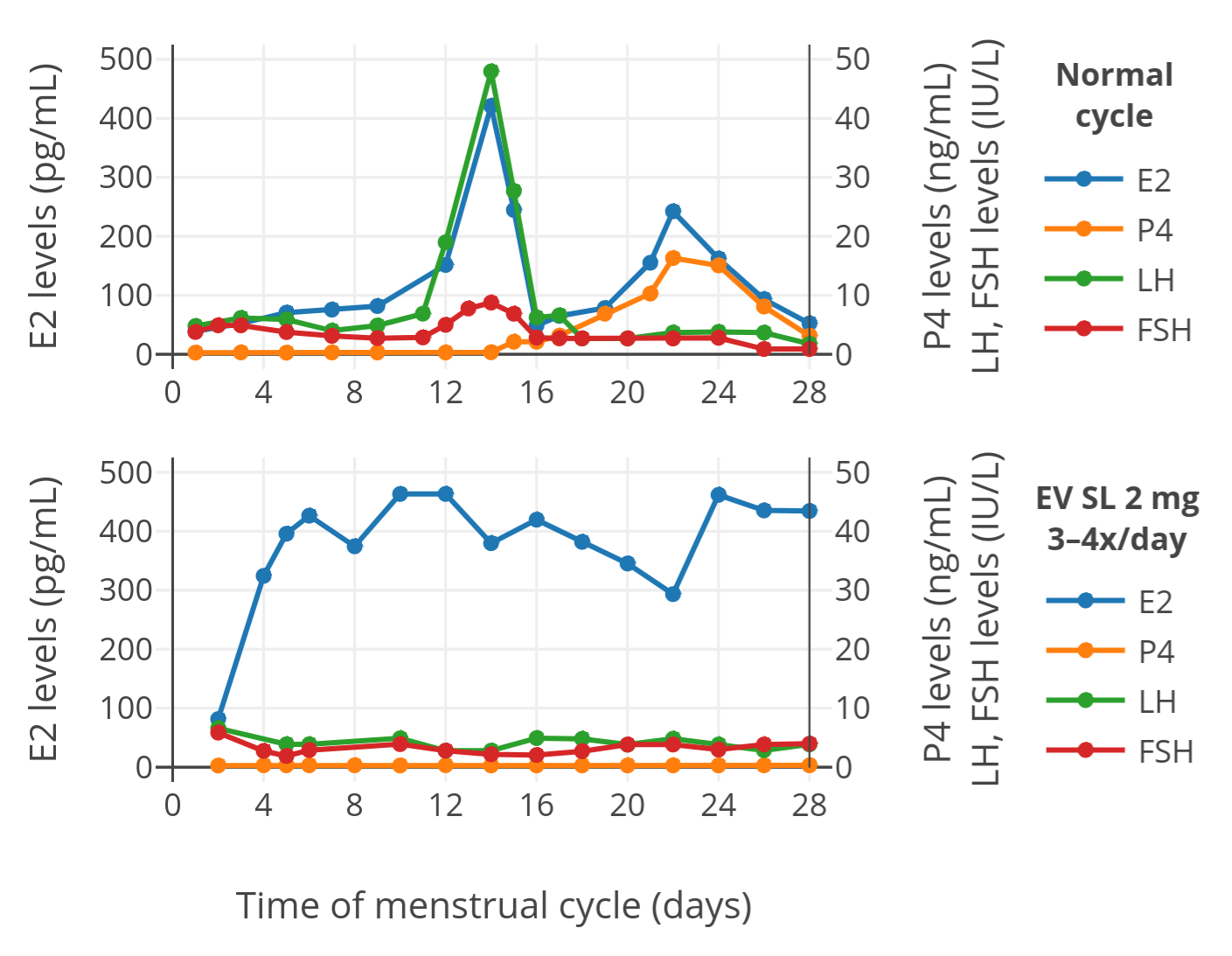
以下是舌下含服 EV 时,激素水平的结果(上图表示参照组的一个月经周期;下图为含服 EV 组的一个周期):
|
||||
|
||||
<section class="box">
|
||||
|
||||
|
||||
|
||||
**图 1**:每日舌下含服 3\~4 次 2mg EV 片(补佳乐,已微粉化)的绝经前妇女之激素水平(下图),包括雌二醇 (E2)、孕酮 (P4)、促黄体生成素 (LH) 与促卵泡激素 (FSH)。上图为参照组的正常月经周期。服药后在何时采血并未明确说明。
|
||||
|
||||
</section>
|
||||
|
||||
如上图所示,舌下含服 EV 组妇女的雌二醇水平,显著高于处于正常周期的参照组。此外,其它激素的水平也被抑制;这是高水平的雌二醇对下丘脑-垂体-性腺轴之负反馈,并对激素产生之抑制所致。该发现表明,舌下含服的 EV 被很好地吸收了,并可达到和含服雌二醇相似的高雌二醇水平。换句话说,尽管尚无直接对比,但显然,经舌下含服 EV 是一种和含服雌二醇类似的高效的给药方式。
|
||||
|
||||
值得一提的是,在韩国,已有至少一座性别肯定激素治疗(GAHT)诊所报告了女性倾向跨性别者使用舌下含服的 EV。当地医师在近期一份论文里 ([Lim et al., 2019](https://doi.org/10.5468/ogs.2019.62.1.46)) 简要描述了使用舌下含服 EV 的经验,以及未使用口服雌二醇而选择它的理论依据。尽管他们并未提供实际的药代动力学数据,但基于其经验,显然舌下含服是一种行之有效的服用 EV 之途径。
|
||||
|
||||
## 雌二醇片与 EV 片的剂型,及其舌下含服之障碍 {#formulation-of-estradiol-and-estradiol-valerate-tablets-and-implications-for-sublingual-use}
|
||||
|
||||
### 口服用 EV 片的微粉化 {#physicochemical-properties-of-estradiol-versus-estradiol-valerate}
|
||||
|
||||
微粉化是一种将固体颗粒变得更小的生产过程。微粉化可修改药物的吸收速率,从而改变其药代动力学特性。口服用的雌二醇片原先并未被微粉化,其雌激素效力相较今日也更低;但到了 1970 年代,微粉化的雌二醇片开始推出,并取代了早前的剂型。事实表明,将雌二醇晶体微粉化为特定大小之颗粒,使得雌二醇片的吸收率及生物利用度翻了几番 ([维基百科](https://en.wikipedia.org/wiki/Pharmacokinetics_of_estradiol#Absorption_and_bioavailability))。现今市面上所有雌二醇片皆可视为已经过微粉化。
|
||||
|
||||
一些问题浮出了水面:诸如微粉化会给舌下含服(而非口服)像雌二醇、EV 之类的激素类药品带来什么影响,以及 EV 片是否也同样经过了微粉化等。关于微粉化在舌下含服时的吸收率、药代动力学特性的方面上对雌二醇(酯)以及孕酮、睾酮等激素的影响,迄今尚未有相关研究,因此其影响不明。不过,一篇文献摘录 (Sayeed & Ashraf, 2014) 提及时,认为微粉化也许提高了舌下含服的吸收速率与程度,类似于口服,因此其影响应该很重要:
|
||||
|
||||
> 经舌下含服的药物通常溶解度很低。因此,为提高溶解性,减小并控制(药物有效成分的)颗粒体积便至关重要。这项参数对所有溶解度低的药物皆乃关键。然而,为舌下含服的药物,需控制愈发小的颗粒体积,以维持药物在有限溶解与吸收窗口之内的可复现的质量及性能。
|
||||
|
||||
至于 EV 片是否经过了微粉化:一些剂型在其包装或厂家资料上明确标示已经过微粉化 ([示例](https://imgur.com/a/onfp0yO)),而其它剂型则未标示。在 Serhal 和 Craft (“补佳乐”) 的研究以及一些其它研究 (例如 Devroey & Pados, 1998) 当中,所用 EV 片皆明确标注已被微粉化。考虑到现今所有口服用 EV 片在相似用量下的明显类似的临床特性,可以认为,所有口服 EV 片之剂型均已被微粉化,只不过有些并未标明罢了。事实上,在 1960 年代末期推出的原始形态的 EV 片,就被指出已微粉化。有一种可能性不大的说法是,微粉化或许并不会影响口服 EV 之特性,就像其之于口服雌二醇那样。
|
||||
|
||||
加州大学旧金山分校 (UCSF) 的《跨性别护理指南》指出,只有经微粉化的雌二醇片可被用于舌下含服,暗示并非所有雌二醇片皆已微粉化 ([Deutsch, 2016](https://transcare.ucsf.edu/sites/transcare.ucsf.edu/files/Transgender-PGACG-6-17-16.pdf#page=26))。该观点可能也适用于 EV 片。然而,正如本文所述,目前这些观点尚缺乏依据。
|
||||
|
||||
### 雌二醇与 EV 的物化特性 {#physicochemical-properties-of-estradiol-versus-estradiol-valerate}
|
||||
|
||||
作为含有脂肪酸(也即戊酸)的酯,EV 的脂溶性较雌二醇更高。脂溶性已知可改变药物舌下、面颊含服时的吸收率 ([Smart, 2005](https://doi.org/10.1517/17425247.2.3.507); [Batheja, Thakur, & Michniak, 2006](https://doi.org/10.1201/9781420004816-17))。关于雌二醇与 EV 在脂溶性之差异如何影响其舌下含服时的药代动力学,迄今尚未得到研究。因此,其带来的疗效如何也不清楚。不过,已知在口腔黏膜及唾液内,存在可以将 EV 之类的有机酸酯分解为结合前之形态的酯酶。这也许能够缩小通过舌下、面颊含服的雌二醇与 EV 在物化特性上的差异。
|
||||
|
||||
雌二醇与 EV 的另一项差别在于,作为一种酯,后者的摩尔质量更大,因此相同用量下后者提供的雌二醇更少。EV 的摩尔质量是雌二醇的 131%;这意味着,EV 内雌二醇约占其质量的 76%。这项差异在对口服雌二醇与 EV 的药代动力学研究上已有体现,在相同用量下,口服 EV 在雌二醇水平上要低 25%。这或许也适用于通过其它途径的给药,包括舌下含服。因此,为达到和服用雌二醇相同的雌二醇水平、与雌激素效力,使用 EV 可能需要稍微提高用量(例如 2mg 对应 1.5mg 雌二醇)。
|
||||
|
||||
### EV 片的剂型与溶解 {#formulation-and-dissolution-of-oral-estradiol-valerate-tablets}
|
||||
|
||||
口服用雌二醇片与 EV 片被设计用于口服,而非舌下含服。其中一些片剂舌下含服的表现尚佳;不过在剂型上,例如包衣、成分与辅料,皆有所区别。一篇文献摘录 ([Sayeed & Ashraf, 2014](https://web.archive.org/web/20201111205841/https://www.pharmtech.com/view/considerations-developing-sublingual-tablets-overview)) 认为,这种差异有可能会影响剂型舌下含服时的特性:
|
||||
|
||||
> 通常,舌下含服的片剂在口腔内分裂并溶解的情况,是有别于口服片剂的。其它一些特制的口服片剂,诸如缓释片、肠溶片等,也可能会在胃中释放部分药剂。与此相反,舌下含服的片剂被设计为可在口腔、舌下完全分裂并溶解。
|
||||
|
||||
有一种特殊情况是:雌二醇、EV 的不同口服片剂,溶解速率或有明显差异。一项关于舌下含服口服用雌二醇片的研究,报告了其在 1\~2 分钟内溶解了 ([Burnier, 1981](https://doi.org/10.1016/0002-9378(81)90101-0))。传闻在美国有一种仿制的糖包衣的雌二醇口服片 ([照片](https://img.medscapestatic.com/pi/features/drugdirectory/octupdate/BRR08870.jpg)),情况与此类似,舌下含服时在数分钟内即溶解以至消失。不过,一些女性倾向跨性别者在 Reddit 等社交平台上披露,她们的片剂在舌下、或面颊内溶解用了更长时间。例如,有人报告其服用的 EV 片(“补佳乐”)用了约一小时的时间才完全溶解 (Reddit [来源一](https://reddit.com/r/MtFHRT/comments/ad0uhp/comment/eg5oyks/); [来源二](https://reddit.com/r/MtFHRT/comments/9h8s04/comment/e8mz1bk/))。尽管如此,她们仍报告了采用单服 EV(面颊含服)疗法时达到了高水平的雌二醇浓度,以及对睾酮的强力压制。总之,基于其剂型,雌二醇片与 EV 片的溶解速率会有差异;而且其中部分剂型相对会更适合舌下含服。如果 _(某品牌)_ 片剂舌下含服时溶解得更慢,也许可以考虑换用其它品牌。
|
||||
|
||||
## 应该选择含服雌二醇片,还是 EV 片? {#estradiol-or-estradiol-valerate-for-sublingual-use}
|
||||
|
||||
相较 EV 片而言,对于雌二醇片的舌下含服,相关调查与描述丰富得多。因此,选择含服不确定性更少的雌二醇片,而非 EV 片,不失为明智之举。不过,在某些市场上,并不一定会有雌二醇片,而 EV 片则会被作为处方开出;雌二醇片也可能出于其它原因而不大适合选用。就这点而言,基于已有文献,显然舌下含服 EV 片是一种和舌下含服雌二醇相似的、高效的雌二醇给药方式,有需要时不妨一用。
|
||||
|
||||
## 参考文献 {#references}
|
||||
|
||||
- Batheja, P., Thakur, R., & Michniak, B. (2006). Basic Biopharmaceutics of Buccal and Sublingual Absorption. In Touitou, E., & Barry, B. W. (Eds.). _Enhancement in Drug Delivery_ (pp. 175–202). Boca Raton/London/New York: CRC Press. \[[Google Scholar](https://scholar.google.com/scholar?cluster=8652202749142820648)\] \[[Google Books](https://books.google.com/books?id=t0L_zFoXmjkC&pg=PA175)\] \[DOI:<https://doi.org/10.1201/9781420004816-17>\] \[[PDF](https://files.transfemscience.org/pdfs/Batheja,%20Thakur,%20&%20Michniak%20(2006)%20-%20Basic%20Biopharmaceutics%20of%20Buccal%20and%20Sublingual%20Absorption%20%5BIn%20Touitou%20&%20Barry%20-%20Enhancement%20in%20Drug%20Delivery%20(pp.%20175%E2%80%93202)%5D.pdf)\]
|
||||
- Burnier, A. M., Martin, P. L., Yen, S. S., & Brooks, P. (1981). Sublingual absorption of micronized 17β-estradiol. _American Journal of Obstetrics and Gynecology_, _140_(2), 146–150. \[DOI:<https://doi.org/10.1016/0002-9378(81)90101-0>\]
|
||||
- Deutsch, M. B. (2016). Overview of feminizing hormone therapy. In Deutsch, M. B. (Ed.). _Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People, 2nd Edition_ (pp. 26–48). San Francisco: University of California, San Francisco/UCSF Transgender Care. \[[Google Scholar](https://scholar.google.com/scholar?cluster=14884919836847089083)\] \[[URL](https://transcare.ucsf.edu/guidelines/feminizing-hormone-therapy)\] \[[PDF](https://transcare.ucsf.edu/sites/transcare.ucsf.edu/files/Transgender-PGACG-6-17-16.pdf#page=26)\]
|
||||
- Devroey, P., & Pados, G. (1998). Preparation of endometrium for egg donation. _Human Reproduction Update_, _4_(6), 856–861. \[DOI:<https://doi.org/10.1093/humupd/4.6.856>\]
|
||||
- Lim, H. H., Jang, Y. H., Choi, G. Y., Lee, J. J., & Lee, E. S. (2019). Gender affirmative care of transgender people: a single center’s experience in Korea. _Obstetrics & Gynecology Science_, _62_(1), 46–55. \[DOI:<https://doi.org/10.5468/ogs.2019.62.1.46>\]
|
||||
- Rathbone, M. J., Drummond, B. K., & Tucker, I. G. (1994). The oral cavity as a site for systemic drug delivery. _Advanced Drug Delivery Reviews_, _13_(1–2), 1–22. \[DOI:<https://doi.org/10.1016/0169-409X(94)90024-8>\]
|
||||
- Sayeed, V. A., & Ashraf, M. (2014). Considerations in Developing Sublingual Tablets—An Overview. _Pharmaceutical Technology_, _38_(11), 34–72. \[[Google Scholar](https://scholar.google.com/scholar?cluster=1357039233409295184)\] \[[URL](https://web.archive.org/web/20201111205841/https://www.pharmtech.com/view/considerations-developing-sublingual-tablets-overview)\] \[[PDF](https://files.transfemscience.org/pdfs/Sayeed%20&%20Ashraf%20(2014)%20-%20Considerations%20in%20Developing%20Sublingual%20Tablets%E2%80%94An%20Overview%20-%20Pharmaceutical%20Technology.pdf)\]
|
||||
- Serhal, P., & Craft, I. (1989). Oocyte donation in 61 patients. _The Lancet_, _333_(8648), 1185–1187. \[DOI:<https://doi.org/10.1016/S0140-6736(89)92762-1>\]
|
||||
- Serhal, P. (1990). Oocyte donation and surrogacy. _British Medical Bulletin_, _46_(3), 796–812. \[DOI:<https://doi.org/10.1093/oxfordjournals.bmb.a072432>\]
|
||||
- Smart, J. D. (2005). Buccal drug delivery. _Expert Opinion on Drug Delivery_, _2_(3), 507–517. \[DOI:<https://doi.org/10.1517/17425247.2.3.507>\]
|
||||
- Yamahara, H., & Lee, V. H. (1993). Drug metabolism in the oral cavity. _Advanced Drug Delivery Reviews_, _12_(1–2), 25–39. \[DOI:<https://doi.org/10.1016/0169-409X(93)90039-7>\]
|
||||
Binary file not shown.
|
After Width: | Height: | Size: 268 KiB |
|
|
@ -0,0 +1,188 @@
|
|||
---
|
||||
title: 关于女性倾向跨性别者舌下含服戊酸雌二醇片剂的简介
|
||||
linkTitle: 舌下含服戊酸雌二醇的可行性
|
||||
description: 戊酸雌二醇的舌下含服途径尽管鲜有研究,但其与含服雌二醇同样有效,也可作为口服途径的替代。
|
||||
author: Aly
|
||||
published: 2019-01-05
|
||||
updated: 2022-11-28
|
||||
translated: 2023-03-30
|
||||
translators:
|
||||
- Bersella AI
|
||||
tags:
|
||||
- 雌激素
|
||||
- 用药途径与剂量
|
||||
trackHash: 43539e195ce7f91fa48a5c855392656dc179226d
|
||||
keywords: [补佳乐, 戊酸雌二醇片, 舌下含服, 用法用量]
|
||||
---
|
||||
|
||||
## 译者按 {#notice}
|
||||
|
||||
1. **<u>⚠免责声明:本文不构成任何医疗、处方建议。如有医疗需要,应于专业医师指导下进行。</u>**
|
||||
1. 因译者能力所限,部分术语之翻译或有纰漏,烦请指正。
|
||||
|
||||
---
|
||||
|
||||
## 摘要 {#abstract--tldr}
|
||||
|
||||
> 口服用雌二醇片可经舌下含服,此举相较口服可达到更高的生物利用度,以及雌二醇水平。因此,舌下含服雌二醇在女性倾向跨性别者当中被广泛采用。在世界部分地区也有戊酸雌二醇口服片,常有女性倾向跨性别者咨询其被舌下含服时是否也会高效吸收。\
|
||||
> 这方面的研究很有限,尚无二者的直接对比;不过至少,已有一项在顺性别女性当中进行的研究表明,戊酸雌二醇片舌下含服时可引起较高的雌二醇水平,以及和雌二醇片类似的对睾酮之抑制。此外,已有至少一座性别肯定激素治疗诊所报告了女性倾向跨性别者使用舌下含服的戊酸雌二醇。因此,舌下含服戊酸雌二醇应该是跟舌下含服雌二醇相似的一种高效给药途径。\
|
||||
> 二者之间有一处不同:戊酸雌二醇的雌二醇含量更少,故所需摄入量也略多。此外,雌二醇片与戊酸雌二醇片并非为舌下含服而准备与设计(尽管很有效),其剂型亦有不同。在临床特性上,*(不同剂型)* 可能的影响尚未可知。不过显然,不同剂型的片剂舌下含服所需吸收时间会有明显差别;因此,某些剂型的雌二醇片和戊酸雌二醇片会更适合用于舌下含服。另外,这些剂型的一些性状,例如微粉化、亲油性等,对其药代动力学的影响也会有异,而这尚未得到研究。\
|
||||
> 由于有关舌下含服雌二醇的资料远比戊酸雌二醇要多,不确定性更少,应建议尽可能使用舌下含服雌二醇。不过如有需要,舌下含服戊酸雌二醇对于女性倾向跨性别者也会很有效。
|
||||
|
||||
## 前言 {#introduction}
|
||||
|
||||
[雌二醇][wiki1]片[如诺坤复(Estrofem)、Estrace 等]被设计用于[口服][wiki2](经口吞服),口服也是其最普遍的用途。然而,作为通常的口服途径之替代,雌二醇片也被用于[舌下含服][wiki3],或[含于面颊][wiki4]或嘴唇、牙龈处。舌下、面颊含服雌二醇片相较口服,可达到更高的[生物利用度][wiki5],以及雌二醇水平<sup>([Sam, 2021][S21-SET]; [维基百科][wiki6-sa]; [图表][graph1]; [维基百科][wiki6-ba])</sup>。\
|
||||
女性倾向跨性别者在激素治疗当中,常采用舌下含服雌二醇。在一些国家(如许多欧洲国家),口服雌二醇会以[戊酸雌二醇][wiki7]片[EV,品牌有“补佳乐”(Progynova)等]的形式提供。经常有女性倾向跨性别者咨询,**戊酸雌二醇片是否也能像雌二醇片那样被舌下含服,以及二者是否会有差异**。本文旨在探讨并聚焦于这些问题。
|
||||
|
||||
## 戊酸雌二醇舌下含服之有效性 {#effectiveness-of-sublingual-estradiol-valerate}
|
||||
|
||||
戊酸雌二醇是一种[雌二醇酯][wiki8],是雌二醇的[前体][wiki9]。雌二醇酯本身在被转换成雌二醇前,并不具备[药理活性][wiki10]。戊酸雌二醇及其它雌二醇酯,会被各种[酯酶][wiki12] [裂解][wiki11]为雌二醇。这些酯[酶][wiki13]遍布全身;从雌二醇酯到雌二醇的代谢过程,除肝脏外,也见于血液和其它组织中 ([维基百科][wiki7-pk])。因此,形如戊酸雌二醇的雌二醇酯,可以不经过肝脏的[首过效应][wiki15](口服后转换为雌二醇时会发生)。综上,对于非口服方式(如舌下含服,以及[肌注][wiki6-ii])的戊酸雌二醇,其之转换不会成为一种障碍。
|
||||
|
||||
关于舌下含服(而非口服)雌二醇片的研究很有限,而针对戊酸雌二醇片的研究则严重缺失。至今尚无在舌下含服雌二醇片与戊酸雌二醇片之间进行的直接比较。因此,关于舌下含服戊酸雌二醇在[药代动力学上][wiki16](例如生物利用度、雌二醇水平、雌二醇[浓度—时间曲线][wiki17])相较雌二醇片表现如何,我们暂无可靠数据。
|
||||
|
||||
只有一项研究调查并描述了戊酸雌二醇片的舌下含服。这项研究对一组每日以口服用戊酸雌二醇片(“补佳乐”品牌)进行 3\~4 次舌下含服的[绝经前][wiki18]顺性别妇女进行了评估。研究发现在以下两篇论文被发表:
|
||||
|
||||
- Serhal, P., & Craft, I. (1989). Oocyte donation in 61 patients. *The Lancet*, *333*(8648), 1185–1187. \[DOI:[10.1016/S0140-6736(89)92762-1][SC89]]
|
||||
- Serhal, P. (1990). Oocyte donation and surrogacy. *British Medical Bulletin*, *46*(3), 796–812. \[DOI:[10.1093/oxfordjournals.bmb.a072432][S90]]
|
||||
|
||||
以下是舌下含服戊酸雌二醇时,激素水平的结果(上图表示参照组的一个[月经周期][wiki19];下图为含服戊酸雌二醇组的一个周期):
|
||||
|
||||
<section class="box">
|
||||
|
||||
![此图来自维基百科][fig1]
|
||||
|
||||
**图 1**:每日舌下含服 3\~4 次 2 mg 戊酸雌二醇片(补佳乐,已微粉化)的绝经前妇女之激素水平(下图),包括雌二醇(E2)、[孕酮][wiki20](P4)、[促黄体激素][wiki21](LH)与[促卵泡激素][wiki22](FSH)。上图为参照组的正常月经周期。服药后在何时采血并未明确说明。
|
||||
|
||||
</section>
|
||||
|
||||
如上图所示,舌下含服戊酸雌二醇组妇女的雌二醇水平,显著高于处于正常周期的参照组。此外,其它激素的水平也被抑制;这是高水平的雌二醇对[下丘脑-垂体-性腺轴][wiki23]之负反馈,并对激素产生之抑制所致。该发现表明,舌下含服的戊酸雌二醇被很好地吸收了,并可达到和含服雌二醇相似的高雌二醇水平。换句话说,尽管尚无直接对比,但显然,经舌下含服戊酸雌二醇是一种和含服雌二醇类似的高效的给药方式。
|
||||
|
||||
值得一提的是,在韩国,已有至少一座性别肯定激素治疗诊所报告了女性倾向跨性别者使用舌下含服的戊酸雌二醇。当地医师在近期一份论文里简要描述了使用舌下含服戊酸雌二醇的经验,以及未使用口服雌二醇而选择它的理论依据<sup>([Lim et al., 2019][L19])</sup>。尽管他们并未提供实际的药代动力学数据,但基于其经验,显然舌下含服是一种行之有效的服用戊酸雌二醇之途径。
|
||||
|
||||
## 雌二醇片与戊酸雌二醇片的剂型,及其舌下含服之障碍 {#formulation-of-estradiol-and-estradiol-valerate-tablets-and-implications-for-sublingual-use}
|
||||
|
||||
### 口服用戊酸雌二醇片的微粉化 {#micronization-of-oral-estradiol-valerate-tablets}
|
||||
|
||||
[微粉化][wiki24]是一种将固体[颗粒][wiki25]变得更小的生产工艺。微粉化可修改药物的[吸收][wiki26]速率,从而改变其药代动力学特性。口服用的雌二醇片原先并未被微粉化,其雌激素[效力][wiki27]相较今日也更低;但到了 1970 年代,微粉化的雌二醇片开始推出,并取代了早前的剂型。事实表明,将雌二醇晶体微粉化为特定大小的颗粒,使得雌二醇片的吸收率及生物利用度翻了几番 ([维基百科][wiki6-aab]; [维基百科][wiki1-h])。现今市面上所有雌二醇片皆可视为已微粉化。
|
||||
|
||||
一些问题浮出了水面:诸如微粉化会给舌下含服(而非口服)像雌二醇、戊酸雌二醇之类的激素类药品带来什么影响,以及戊酸雌二醇片是否也同样经过了微粉化等。关于微粉化在舌下含服时的吸收率、药代动力学特性的方面上对雌二醇(酯)以及[孕酮][wiki28]、[睾酮][wiki29]等激素的影响,迄今尚未有相关研究,因此其影响不明。不过,一篇文献摘录提及时,认为微粉化也许提高了舌下含服的吸收速率与程度,类似于口服,因此其影响应该很重要<sup>([Sayeed & Ashraf, 2014][SA14])</sup>:
|
||||
|
||||
> 经舌下含服的药物通常溶解度很低。因此,为提高溶解性,减小并控制(药物有效成分的)颗粒体积便至关重要。这项参数对所有溶解度低的药物皆乃关键。然而,为舌下含服的药物,需控制愈发小的(有效成分)颗粒体积,以维持药物在有限溶解与吸收窗口之内的可复现的质量及性能。
|
||||
|
||||
至于戊酸雌二醇片是否经过了微粉化:一些剂型在其包装或厂家资料上明确标示已经过微粉化<sup>([示例][img1])</sup>,而其它剂型则未标示。在 Serhal 和 Craft (“补佳乐”) 的研究以及一些其它研究<sup>(例如 [Devroey & Pados, 1998][DP98])</sup>当中,所用戊酸雌二醇片皆明确标注已被微粉化。考虑到现今所有口服用戊酸雌二醇片在相似用量下的明显类似的临床特性,可以认为,所有口服戊酸雌二醇片之剂型均已被微粉化,只不过有些并未标明罢了。事实上,最早在 1960 年代末推出的戊酸雌二醇片,就被指出已微粉化<sup>([维基百科][wiki1-h])</sup>。有一种可能性不大的说法是,微粉化或许并不会影响口服戊酸雌二醇之特性,就像其之于口服雌二醇那样。
|
||||
|
||||
加州大学旧金山分校(UCSF)的《跨性别护理指南》指出,只有经微粉化的雌二醇片可被用于舌下含服,暗示并非所有雌二醇片皆已微粉化<sup>([Deutsch, 2016][D16])</sup>。该观点可能也适用于戊酸雌二醇片。然而,正如本文所述,目前这些观点尚缺乏依据。
|
||||
|
||||
### 雌二醇与戊酸雌二醇的物化特性 {#physicochemical-properties-of-estradiol-versus-estradiol-valerate}
|
||||
|
||||
作为含有[脂肪酸][wiki31](也即[戊酸][wiki32])的酯,戊酸雌二醇的[脂溶性][wiki30]较雌二醇更高。脂溶性已知可改变药物舌下、面颊含服时的吸收率<sup>([Smart, 2005][S05]; [Batheja, Thakur, & Michniak, 2006][BTM06])</sup>。关于雌二醇与戊酸雌二醇在脂溶性之差异如何影响其舌下含服时的药代动力学,迄今尚未得到研究。因此,其[物化][wiki33]特性带来的疗效差异也不清楚。不过,已知在口腔黏膜及唾液内,存在可以将戊酸雌二醇之类的[有机酸][wiki34]酯分解为结合前之形态的酯酶<sup>([Yamahara & Lee, 1993][YL93]; [Rathbone, Drummond, & Tucker, 1994][RDT94])</sup>。这也许能够缩小通过舌下、面颊含服的雌二醇与戊酸雌二醇在物化特性上的差异。
|
||||
|
||||
雌二醇与戊酸雌二醇的另一项差别在于:作为一种酯,后者的[分子质量][wiki35]更大,因此相同剂量下后者提供的雌二醇更少。戊酸雌二醇的分子质量是雌二醇的 131%;这意味着,戊酸雌二醇内含雌二醇约占其质量的 76%<sup>([表格][table1])</sup>。这项差异在对口服雌二醇与戊酸雌二醇的药代动力学研究上已有体现,在相同剂量下,口服戊酸雌二醇在雌二醇水平上要低 25%<sup>([维基百科][wiki7])</sup>。这或许也适用于通过其它途径的给药,包括舌下含服。因此,为达到和服用雌二醇相同的雌二醇水平、与雌激素效力,使用戊酸雌二醇可能需要稍微提高用量(例如 2mg 对应 1.5mg 雌二醇)<sup>([Sam, 2021][S21-SET])</sup>。
|
||||
|
||||
### 戊酸雌二醇片的剂型与溶解 {#formulation-and-dissolution-of-oral-estradiol-valerate-tablets}
|
||||
|
||||
雌二醇片与戊酸雌二醇片被设计用于口服,而非舌下含服。其中一些片剂舌下含服的表现尚佳;不过在剂型上,例如[包衣][wiki36]、[成分][wiki37]与[辅料][wiki38],皆有所区别。一篇文献摘录认为,这种差异有可能会影响剂型舌下含服时的特性<sup>([Sayeed & Ashraf, 2014][SA14])</sup>:
|
||||
|
||||
> 通常,舌下含服的片剂在口腔内分裂并溶解的情况,是有别于口服片剂的。……\
|
||||
> 其它一些特制的口服片剂,诸如缓释片、肠溶片等,也可能会在胃中释放部分药剂。与此相反,舌下含服的片剂被设计为可在口腔、舌下完全分裂并溶解。
|
||||
|
||||
这里要注意一个问题:雌二醇、戊酸雌二醇的不同口服片剂,溶解速率或有明显差异。一项关于舌下含服口服用雌二醇片的研究,报告了其在 1\~2 分钟内溶解了<sup>([Burnier, 1981][B81])</sup>。传闻在美国有一种仿制的糖包衣的雌二醇口服片<sup>([照片][img2])</sup>,情况与此类似,舌下含服时在数分钟内即溶解并消失。不过,一些女性倾向跨性别者在 Reddit 等社交平台上披露,她们的片剂在舌下、或面颊内溶解用了更长时间,例如一个小时左右。\
|
||||
无论如何,基于其剂型,雌二醇片与戊酸雌二醇片的溶解速率会有差异;而且其中部分剂型相对会更适合舌下含服。如果 _(某品牌)_ 片剂舌下含服时溶解得慢,可以考虑换用其它品牌。
|
||||
|
||||
## 应该选择含服雌二醇片,还是戊酸雌二醇片? {#estradiol-or-estradiol-valerate-for-sublingual-use}
|
||||
|
||||
相较戊酸雌二醇片而言,舌下含服雌二醇片的相关调查与描述丰富得多。因此,选择含服不确定性更少的雌二醇片,而非戊酸雌二醇片,不失为明智之举。不过,在某些地域的市场上,并不一定会有雌二醇片,取而代之的是戊酸雌二醇片;雌二醇片也可能出于其它原因而不大适合选用。就这点而言,基于已有文献,显然舌下含服戊酸雌二醇片是一种和舌下含服雌二醇相似的、高效的雌二醇给药方式,有需要时不妨一用。
|
||||
|
||||
## 参考文献 {#references}
|
||||
|
||||
- Batheja, P., Thakur, R., & Michniak, B. (2006). Basic Biopharmaceutics of Buccal and Sublingual Absorption. In Touitou, E., & Barry, B. W. (Eds.). *Enhancement in Drug Delivery* (pp. 175–202). Boca Raton/London/New York: CRC Press. \[[Google 学术][BTM06-GS]] \[[Google 阅读][BTM06-GS]] \[DOI:[10.1201/9781420004816-17][BTM06]] \[[PDF 文档][BTM06-PDF]]
|
||||
- Burnier, A. M., Martin, P. L., Yen, S. S., & Brooks, P. (1981). Sublingual absorption of micronized 17β-estradiol. *American Journal of Obstetrics and Gynecology*, *140*(2), 146–150. \[DOI:[10.1016/0002-9378(81)90101-0][B81]]
|
||||
- Deutsch, M. B. (2016). Overview of feminizing hormone therapy. In Deutsch, M. B. (Ed.). *Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People, 2nd Edition* (pp. 26–48). San Francisco: University of California, San Francisco/UCSF Transgender Care. \[[Google 学术][D16-GS]] \[[网址][D16-URL]] \[[PDF 文档][D16]]
|
||||
- Devroey, P., & Pados, G. (1998). Preparation of endometrium for egg donation. *Human Reproduction Update*, *4*(6), 856–861. \[DOI:[10.1093/humupd/4.6.856][DP98]]
|
||||
- Lim, H. H., Jang, Y. H., Choi, G. Y., Lee, J. J., & Lee, E. S. (2019). Gender affirmative care of transgender people: a single center’s experience in Korea. *Obstetrics & Gynecology Science*, *62*(1), 46–55. \[DOI:[10.5468/ogs.2019.62.1.46][L19]]
|
||||
- Rathbone, M. J., Drummond, B. K., & Tucker, I. G. (1994). The oral cavity as a site for systemic drug delivery. *Advanced Drug Delivery Reviews*, *13*(1–2), 1–22. \[DOI:[10.1016/0169-409X(94)90024-8][RDT94]]
|
||||
- Sayeed, V. A., & Ashraf, M. (2014). Considerations in Developing Sublingual Tablets—An Overview. *Pharmaceutical Technology*, *38*(11), 34–72. \[[Google 学术][SA14-GS]] \[[网址][SA14]] \[[PDF 文档][SA14-PDF]]
|
||||
- Serhal, P., & Craft, I. (1989). Oocyte donation in 61 patients. *The Lancet*, *333*(8648), 1185–1187. \[DOI:[10.1016/S0140-6736(89)92762-1][SC89]]
|
||||
- Serhal, P. (1990). Oocyte donation and surrogacy. *British Medical Bulletin*, *46*(3), 796–812. \[DOI:[10.1093/oxfordjournals.bmb.a072432][S90]]
|
||||
- Smart, J. D. (2005). Buccal drug delivery. *Expert Opinion on Drug Delivery*, *2*(3), 507–517. \[DOI:[10.1517/17425247.2.3.507][S05]]
|
||||
- Yamahara, H., & Lee, V. H. (1993). Drug metabolism in the oral cavity. *Advanced Drug Delivery Reviews*, *12*(1–2), 25–39. \[DOI:[10.1016/0169-409X(93)90039-7][YL93]]
|
||||
|
||||
---
|
||||
|
||||
## 译文修订记录 {#revision}
|
||||
|
||||
```csv
|
||||
时间,备注
|
||||
2022 年 2 月 18 日,首次翻译。
|
||||
2023 年 3 月 30 日,第一次修订,优化个别叙述,补齐链接。
|
||||
```
|
||||
|
||||
[wiki1]: https://en.wikipedia.org/wiki/Estradiol_%28medication%29
|
||||
[wiki1-h]: https://en.wikipedia.org/wiki/Estradiol_%28medication%29#History
|
||||
[wiki2]: https://en.wikipedia.org/wiki/Oral_administration
|
||||
[wiki3]: https://en.wikipedia.org/wiki/Sublingual_administration
|
||||
[wiki4]: https://en.wikipedia.org/wiki/Buccal_administration
|
||||
[wiki5]: https://en.wikipedia.org/wiki/Bioavailability
|
||||
[wiki6-sa]: https://en.wikipedia.org/wiki/Pharmacokinetics_of_estradiol#Sublingual_administration
|
||||
[graph1]: https://en.wikipedia.org/wiki/Template:Hormone_levels_with_sublingual_estradiol
|
||||
[wiki6-ba]: https://en.wikipedia.org/wiki/Pharmacokinetics_of_estradiol#Buccal_administration
|
||||
[wiki6-ii]: https://en.wikipedia.org/wiki/Pharmacokinetics_of_estradiol#Intramuscular_injection
|
||||
[wiki6-aab]: https://en.wikipedia.org/wiki/Pharmacokinetics_of_estradiol#Absorption_and_bioavailability
|
||||
[wiki7]: https://en.wikipedia.org/wiki/Estradiol_valerate
|
||||
[wiki7-pk]: https://en.wikipedia.org/wiki/Estradiol_valerate#Pharmacokinetics
|
||||
[wiki8]: https://en.wikipedia.org/wiki/Estrogen_ester
|
||||
[wiki9]: https://en.wikipedia.org/wiki/Prodrug
|
||||
[wiki10]: https://en.wikipedia.org/wiki/Biological_activity
|
||||
[wiki11]: https://en.wikipedia.org/wiki/Bond_cleavage
|
||||
[wiki12]: https://en.wikipedia.org/wiki/Esterase
|
||||
[wiki13]: https://en.wikipedia.org/wiki/Enzyme
|
||||
[wiki14]: https://en.wikipedia.org/wiki/Biotransformation
|
||||
[wiki15]: https://en.wikipedia.org/wiki/Liver
|
||||
[wiki16]: https://en.wikipedia.org/wiki/Pharmacokinetics
|
||||
[wiki17]: https://en.wiktionary.org/wiki/concentration-time_curve
|
||||
[wiki18]: https://en.wikipedia.org/wiki/Premenopause
|
||||
[wiki19]: https://en.wikipedia.org/wiki/Menstrual_cycle
|
||||
[fig1]: fig1.png
|
||||
[wiki20]: https://en.wikipedia.org/wiki/Progesterone
|
||||
[wiki21]: https://en.wikipedia.org/wiki/Luteinizing_hormone
|
||||
[wiki22]: https://en.wikipedia.org/wiki/Follicle-stimulating_hormone
|
||||
[wiki23]: https://en.wikipedia.org/wiki/Hypothalamic%E2%80%93pituitary%E2%80%93gonadal_axis
|
||||
[wiki24]: https://en.wikipedia.org/wiki/Micronization
|
||||
[wiki25]: https://en.wikipedia.org/wiki/Particle_size
|
||||
[wiki26]: https://en.wikipedia.org/wiki/Absorption_%28pharmacology%29
|
||||
[wiki27]: https://en.wikipedia.org/wiki/Potency_%28pharmacology%29
|
||||
[wiki28]: https://en.wikipedia.org/wiki/Progesterone_%28medication%29
|
||||
[wiki29]: https://en.wikipedia.org/wiki/Testosterone_%28medication%29
|
||||
[img1]: https://imgur.com/a/onfp0yO
|
||||
[wiki30]: https://en.wikipedia.org/wiki/Lipophilicity
|
||||
[wiki31]: https://en.wikipedia.org/wiki/Fatty_acid
|
||||
[wiki32]: https://en.wikipedia.org/wiki/Valeric_acid
|
||||
[wiki33]: https://en.wiktionary.org/wiki/physicochemical
|
||||
[wiki34]: https://en.wikipedia.org/wiki/Carboxylic_acid
|
||||
[wiki35]: https://en.wikipedia.org/wiki/Molecular_weight
|
||||
[table1]: https://en.wikipedia.org/wiki/Template:Structural_properties_of_selected_estradiol_esters
|
||||
[wiki36]: https://en.wikipedia.org/wiki/Film_coating
|
||||
[wiki37]: https://en.wikipedia.org/wiki/Chemical_composition
|
||||
[wiki38]: https://en.wikipedia.org/wiki/Excipient
|
||||
[img2]: hhttps://web.archive.org/web/20170716044325if_/http://img.medscapestatic.com/pi/features/drugdirectory/octupdate/BRR08870.jpg
|
||||
|
||||
[S21-SET]: https://transfemscience.org/articles/sublingual-e2-transfem/
|
||||
[SC89]: https://doi.org/10.1016/S0140-6736%2889%2992762-1
|
||||
[S90]: https://doi.org/10.1093/oxfordjournals.bmb.a072432
|
||||
[L19]: https://doi.org/10.5468/ogs.2019.62.1.46
|
||||
[SA14]: https://web.archive.org/web/20201111205841/https://www.pharmtech.com/view/considerations-developing-sublingual-tablets-overview
|
||||
[DP98]: https://doi.org/10.1093/humupd/4.6.856
|
||||
[D16]: https://transcare.ucsf.edu/sites/transcare.ucsf.edu/files/Transgender-PGACG-6-17-16.pdf#page=26
|
||||
[S05]: https://doi.org/10.1517/17425247.2.3.507
|
||||
[BTM06]: https://doi.org/10.1201/9781420004816-17
|
||||
[YL93]: https://doi.org/10.1016/0169-409X%2893%2990039-7
|
||||
[RDT94]: https://doi.org/10.1016/0169-409X%2894%2990024-8
|
||||
[B81]: https://doi.org/10.1016/0002-9378%2881%2990101-0
|
||||
|
||||
[BTM06-GS]: https://scholar.google.com/scholar?cluster=8652202749142820648
|
||||
[BTM06-GS]: https://books.google.com/books?id=t0L_zFoXmjkC&pg=PA175
|
||||
[BTM06-PDF]: https://files.transfemscience.org/pdfs/Batheja,%20Thakur,%20&%20Michniak%20%282006%29%20-%20Basic%20Biopharmaceutics%20of%20Buccal%20and%20Sublingual%20Absorption%20[In%20Touitou%20&%20Barry%20-%20Enhancement%20in%20Drug%20Delivery%20%28pp.%20175%E2%80%93202%29].pdf
|
||||
[D16-GS]: https://scholar.google.com/scholar?cluster=14884919836847089083
|
||||
[D16-URL]: https://transcare.ucsf.edu/guidelines/feminizing-hormone-therapy
|
||||
[SA14-GS]: https://scholar.google.com/scholar?cluster=1357039233409295184
|
||||
[SA14-PDF]: https://files.transfemscience.org/pdfs/Sayeed%20&%20Ashraf%20%282014%29%20-%20Considerations%20in%20Developing%20Sublingual%20Tablets%E2%80%94An%20Overview%20-%20Pharmaceutical%20Technology.pdf
|
||||
Loading…
Reference in New Issue