684 lines
95 KiB
Markdown
684 lines
95 KiB
Markdown
---
|
||
title: 低剂量的醋酸环丙孕酮足以最大限度地抑制女性倾向跨性别者的睾酮水平
|
||
linkTitle: 低剂量醋酸环丙孕酮足可高效抑制睾酮
|
||
description: 本文探讨了以更低剂量服用 CPA,并深入论证了低剂量的合理性。
|
||
author: Aly
|
||
published: 2019-07-01
|
||
updated: 2023-03-31
|
||
translated: 2023-04-05
|
||
translators:
|
||
- Bersella AI
|
||
tags:
|
||
- 醋酸环丙孕酮
|
||
- 孕激素
|
||
- 抗雄激素制剂
|
||
- 用药安全
|
||
- 用药途径与剂量
|
||
trackHash: 9647d91f8140784cd1b54bab2d3e2a6552fafa9d
|
||
keywords: [醋酸环丙孕酮, 色谱龙, 抗雄激素, 副作用, 用法用量]
|
||
---
|
||
|
||
## 译者按 {#notice}
|
||
|
||
1. **<u>⚠ 免责声明:本文不构成任何医疗、处方建议。如有医疗需要,应于专业医师指导下进行。</u>**
|
||
1. 因译者能力所限,部分术语之翻译或有纰漏,烦请指正。
|
||
|
||
---
|
||
|
||
## 摘要 {#abstract}
|
||
|
||
> 醋酸环丙孕酮(CPA)是一种孕激素与抗雄制剂,广泛用于女性化激素治疗。其作为孕激素的作用,远甚于作为雄激素受体拮抗剂的作用。一般作为孕激素用于顺性别妇女时,其剂量介乎 1–2 mg/天;作为抗雄制剂时,其剂量介乎 50–300 mg/天。但对于后者,会有强烈的孕激素过量影响,以及与之关联的副作用和风险。\
|
||
> CPA 因其孕激素作用可抑制睾酮水平,故也有拮抗促性腺激素之效应。单服 CPA 时,睾酮水平最大可压制 50% 至 70%;而结合小剂量雌激素服用时,其可完全抑制来自性腺的睾酮分泌,并将睾酮水平降低约 95%——正好落入女性范围。尽管历史上曾有女性倾向跨性别者每日服用 50–100 mg 的 CPA,但目前已知的是,5–10 mg/天的剂量足以最大程度(或接近最大程度)抑制睾酮水平。\
|
||
> CPA 本身通常以 50 mg/片的形式提供。这些片剂可被切药器切开,之后每日或隔日服用一次,这样日均剂量可减至 6.25 至 12.5 mg。低剂量 CPA 不仅可显著减少开销,而且有更好的耐受性及安全性。出于其低剂量仍保持的效用、以及和剂量相关的风险之考量,针对女性倾向跨性别者的 CPA 临床用量正在快速减少。
|
||
|
||
## 前言 {#introduction}
|
||
|
||
本文讨论[醋酸环丙孕酮][wiki1](CPA)的剂量。CPA 是一种孕激素制剂与抗雄制剂,用于女性倾向跨性别者的激素治疗。\
|
||
本文探讨了以更低剂量服用 CPA,并深入论证了低剂量的合理性。
|
||
|
||
如果读者只对推荐剂量感兴趣,可见[推荐剂量]({{< ref "#recommended-dosages" >}})一节。
|
||
|
||
## CPA 之效力、传统剂量与健康风险 {#potency-conventional-dosages-and-health-risks}
|
||
|
||
CPA 是一种强效孕激素,当用于顺性别妇女时,1 mg/天的剂量即可[抑制排卵][wiki32],1–3 mg/天即可[使子宫内膜转化][wiki33] <sup>([维基百科][wiki2-pd]; [表格][table1]; [Endrikat et al., 2011][E11])</sup>。该剂量的 CPA 之效力,相当于处在黄体期的绝经前妇女自然产生的孕酮量(25 mg/天)以及孕酮水平(15 ng/mL)之效力。与此对应,当 CPA 作为孕激素用于顺性别妇女时(例如作为避孕药,或更年期激素疗法制剂),其以每片 1/2 mg 的形式提供<sup>([维基百科][wiki1-af])</sup>。
|
||
|
||
和其孕激素效力相反,CPA 作为雄激素受体拮抗剂之效力弱得多<sup>([维基百科][wiki2-pd])</sup>。作为抗雄制剂,其剂量一般介乎 50–300 mg/天,对于顺性别男女皆如此。对于女性,一般使用 50–100 mg/天以改善受雄激素影响的皮肤与毛发之状况(例如痤疮与多毛症);而对于男性,则一般使用 100–300 mg/天以治疗前列腺癌(若伴随去势手段,则使用 100–200 mg/天;单服 CPA 疗法则需 200–300 mg/天)<sup>([维基百科][wiki1-mu])</sup>。为此,CPA 一般被制成 50mg 或 100mg 的片剂以供服用 <sup>([维基百科][wiki1-af])</sup>。CPA 作为抗雄制剂有双重机制:通过其低剂量下的孕激素作用来抑制睾酮水平,高剂量下还可直接阻止睾酮作用于雄激素受体。
|
||
|
||
在传统临床剂量下,因孕激素效力远甚于雄激素受体拮抗剂效力,CPA 会有强烈的孕激素过量影响。有三篇文献摘录描述了这点:
|
||
|
||
> CPA 与其前身[醋酸氯地孕酮][wiki3]相似,皆为强效孕激素,在 20–30 mg 剂量下即可使子宫内膜转化。……\
|
||
> CPA 每个月为完全达到临床上的雄激素拮抗效果所需剂量的生理效力,应相当于一个月经周期内所产生孕酮之效力的三十倍。CPA 尽管是 _(抗雄)_ 这方面最有用的药物,但并非是理想的抗雄制剂,尤其是,某些副作用还与孕激素的过量摄入有关(而非其雄激素拮抗效应)。……\
|
||
> 在需要 CPA 完全发挥雄激素拮抗效应的场合,产生的诸如疲倦、无力、体重增加等不良反应皆可能与严重过量摄入的孕激素之效力有关。([Hammerstein et al., 1975][H75])
|
||
|
||
> Fixson (1963) 在已摘除卵巢、且事前服用了雌激素的妇女身上试用 CPA;20–30 mg 的剂量可使子宫内膜转化,这说明其为一种强效孕激素。对于月经推迟的试验,其有效剂量不明,但估计应低于 1mg/天<sup>(Miller and Jacobs 1986)</sup>。\
|
||
> 相比于孕激素效力,CPA 的雄激素拮抗效应应被认为相当羸弱;为完全发挥该效应,需要每日服用 100mg,这相当于用于月经周期转化的剂量之三倍<sup>(Hammerstein and Cupceancu 1969)</sup>(值得一提的是,该数值相当于整个月经周期中黄体分泌的孕酮总量)。 ([Hammerstein, 1990][H90])
|
||
|
||
> 在内分泌学性质上,CPA 应具有较强的孕激素效力以及有限的雄激素拮抗效力。……\
|
||
> 关于其孕激素活性,每月(每周期)需要 20-30 mg 以转化受雌激素驱动(oestrogen-primed)的子宫内膜,该剂量和醋酸氯地孕酮以及其它强效孕激素相似。为完全发挥雄激素拮抗效力,每日须至少服用 50-100 mg 的 CPA,此剂量相当于女性在整个月经周期的孕酮暴露量的两到三倍。必须承认,这种孕激素的摄入已严重过量,除非大幅减少其用量以及效力。……\
|
||
> 已有人指出,CPA 并非一种有利于内分泌平衡的药物成分,因为其孕激素效力显著优于雄激素拮抗效力。有一种方法可以避免伴随高剂量反向序贯疗法而生的孕激素的严重过量:就是合并使用低剂量的避孕药和一种纯抗雄制剂(例如游离的[环丙孕酮][wiki4])。……\
|
||
> 必须强调的是,CPA 远不是一种用于多毛症的抗雄疗法的理想药物,因为其孕激素效力过强,局部作用也并不佳。因此,在未来值得为此寻找平衡性更好的抗雄制剂。 ([Hammerstein, 1979][H79])
|
||
|
||
因高剂量 CPA 导致的孕激素摄入的高度过量,以及 CPA 高剂量下产生的已知不良反应与风险之间,存在一定关联<sup>([维基百科][wiki5])</sup>。这些副作用包括:疲倦、抑郁、体重增加、高泌乳素水平<sup>([维基百科][wiki5-hpl])</sup>、良性脑膜瘤<sup>([Aly, 2020][AW20-CM]; [维基百科][wiki5-bt]; [表格 1][table2]; [表格 2][table3])</sup>、血栓<sup>([维基百科][wiki5-bc])</sup>以及心血管问题<sup>([维基百科][wiki5-ce])</sup>等。这些风险和剂量相关;迄今未发现每日 1/2 mg 的 CPA 与此存在关联(但有一项例外,即合用炔雌醇时会提高血栓风险)。CPA 的肝毒性同样与剂量相关:一般当剂量超过 20 mg/天时,肝转化酶水平会有升高;若超过 100 mg/天,有罕见概率出现肝衰竭<sup>([维基百科][wiki5-lt]; [表格][table4])</sup>。低剂量能够尽量减少风险,这就是为何使用尽可能少的 CPA 的根本所在。
|
||
|
||
历史上,曾有女性倾向跨性别者每日服用 50–100 mg 的 CPA<sup>(例如 [Moore, Wisniewski, & Dobs, 2003][MWD03])</sup>。但在 2017 年,美国内分泌学会发布了最新一版针对跨性别者激素治疗的临床实践指南,其中将 CPA 的推荐用量从 50–100 mg/天降至 25–50 mg/天<sup>([Hembree et al., 2017][H17]; [Hembree et al., 2009][H09])</sup>。此举可能是受到了日渐明了的高剂量 CPA 之风险的驱动。不过,新的推荐用量似乎仍远超实际所需。
|
||
|
||
## 低剂量或高剂量 CPA 对睾酮的抑制 {#testosterone-suppression-with-low-and-high-doses}
|
||
|
||
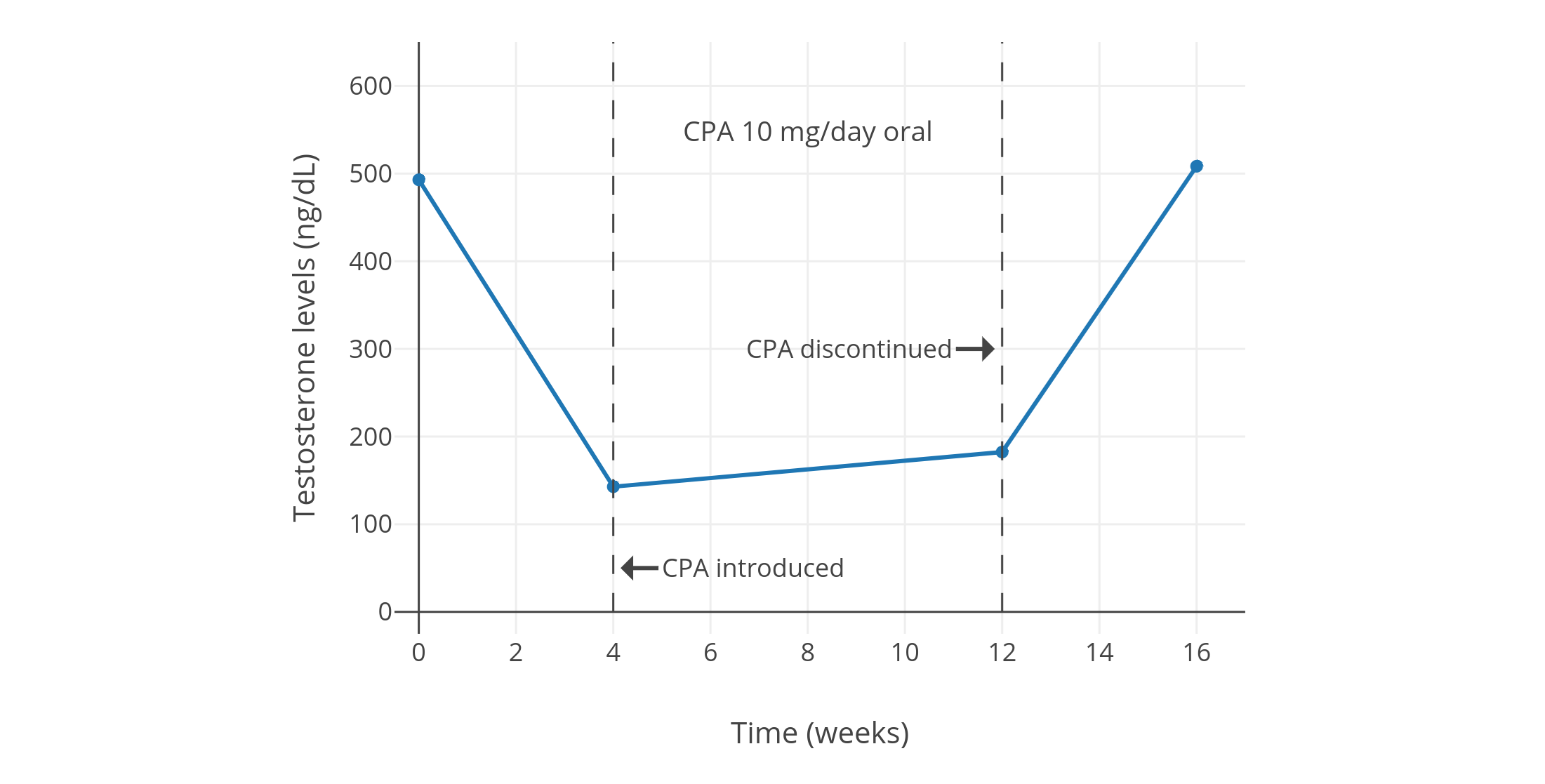
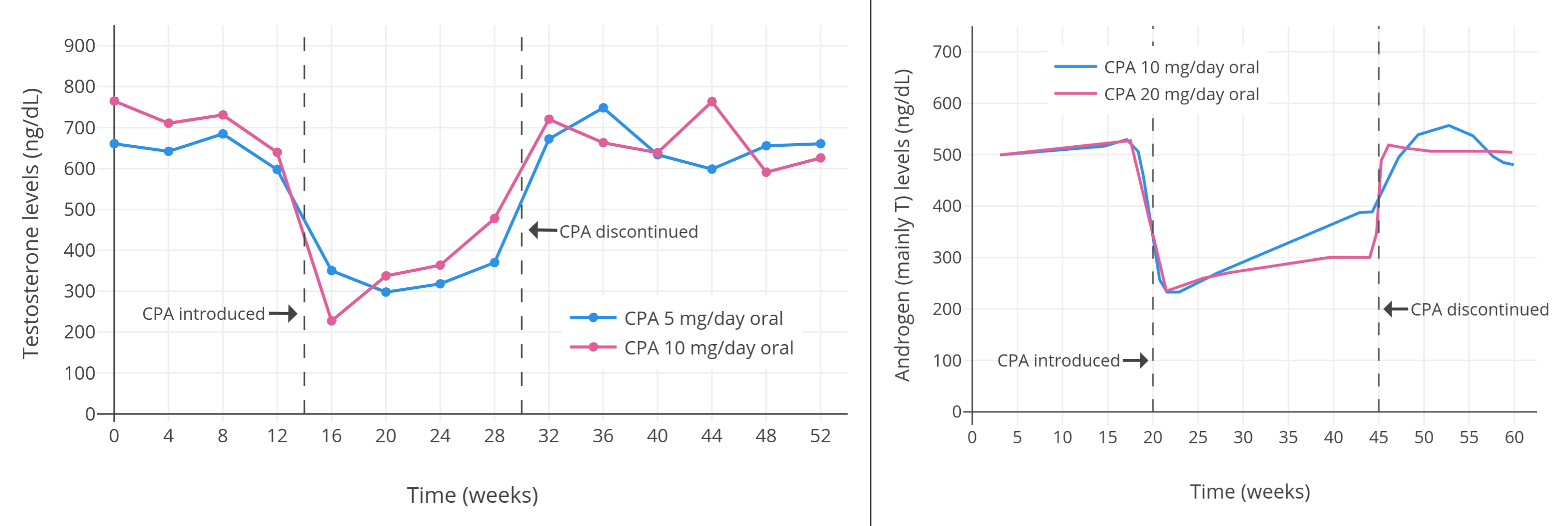
像 CPA 一类的孕激素,可以显著抑制出生指派性别为男、性腺完整的人群当中的睾酮水平。从 1970 年代到 1980 年代初发表的一系列规模较小且质量较低、但值得一提的研究项目发现,健康年轻男性每日服用 5–10 mg 的 CPA,可将睾酮水平抑制 40–70%(见表 1)。其中,一些项目报告了使用 5 mg/天剂量的睾酮抑制程度,和使用 10 mg/天剂量的几乎一致(皆抑制了约 50%)<sup>([Wang & Yeung, 1980][WY80]; [图表][graph1])</sup>;而 10 mg/天剂量的效果则与 20 mg/天相近(皆为约 60–70%)<sup>([Koch et al., 1976][K76]; [Koch et al., 1975][K75]; [图表][graph2])</sup>。同一项目里即使 CPA 剂量加倍,也未能提高睾酮抑制率,这表明实际仅需 5 或 10 mg/天的 CPA 剂量,即可最大程度抑制睾酮。\
|
||
一项近年进行的、使用更现代和更可靠的血清睾酮定量方法的研究项目,发现了 10 mg/天用量的 CPA 可将睾酮水平抑制 66%(从约 600±150 ng/dL 压减至约 185 ng/dL)<sup>([Meriggiola et al., 2002a][M02a]; [图表][graph10])</sup>。\
|
||
与此类似,另一项年份更近的研究发现,10–20 mg/天的 CPA 可将睾酮水平从 431 ng/dL 左右抑制到 149 ng/dL 左右,抑制率 65%;且不同剂量间未发现(疗效)差异。<sup>([Zitzmann et al., 2017][Z17]; [图表][graph9])</sup>
|
||
|
||
<section class="box">
|
||
|
||
**表 1:** 低剂量 CPA(5–30 mg/天)所引起的睾酮等性激素水平的变化
|
||
|
||
| CPA 剂量 | 受试者 | 结果 | 资料来源 |
|
||
|-|-|-|-|
|
||
| 30 mg/天 | 正常男性 5 人 | 睾酮“大幅”减少。<br>未提供具体数值,但有个体的睾酮水平图表。<br>后来对一人试验 5 mg/天剂量,其对精子生成或睾酮的影响不及 30 mg/天。<br>此外还报告了促性腺激素分泌减少。 | [Petry et al. (1972)][P72];<br>[Petry et al. (1970a)][P70a];<br>[Petry et al. (1970b)][P70b];<br>[Petry et al. (1970c)][P70c] |
|
||
| 10 或 20 mg/天 | 25–35 岁健康可生育女性 15 人;<br>其中 7 人剂量 10 mg/天,<br>8 人剂量 20 mg/天 | 两个组别的“雄激素(主要为睾酮)”均下降 60%。<br>{{< abbr "LH" >}} 变化不一,{{< abbr "FSH" >}} 略为下降。<br>未提供具体数值,仅有图表。 | [Koch et al. (1976)][K76];<br>[Koch et al. (1975)][K75] |
|
||
| 0、5 或 10 mg/天 | 20–40 岁健康男性 18 人<br>(分为 3 组,每组 6 人)<br> | 睾酮下降,{{< abbr "LH" >}} 与 {{< abbr "FSH" >}} 不变。<br>未提供具体激素水平或其它细节。 | [Roy et al. (1976)][R76] |
|
||
| 10 mg/天 | 可生育的健康年轻男性 10 人<br>(年龄 21–35 岁,平均 27.2 ± 3.2 岁) | 睾酮初值 495 ± 66 ng/dL,四周后降至 154 ± 23 ng/dL,降幅 70%;<br>十二个月后睾酮 187 ± 38 ng/dL。<br>此外 {{< abbr "DHT" >}} 下降 50%,LH 下降 30%,FSH 下降 40%;而泌乳素升高 75%。<br>还有其它激素水平的数值及图表。 | [Moltz et al. (1980)][M80];<br>[Moltz et al. (1978a)][M78a];<br>[Moltz et al. (1978b)][M78b] |
|
||
| 5 或 10 mg/天 | 20–40 岁健康男性 14 人<br>(每组 7 人)| 两个组别的睾酮均下降。<br>未提供具体激素水平或其它细节。 | [Roy & Chatterjee (1979a)][RC79a] |
|
||
| 10 mg/天 | 32–35 岁正常可生育男性 3 人;<br>单用 CPA 12–18 周,<br>此后与 75 mg/天的美睾酮并用 | 论文未提及睾酮水平。 | [Roy & Chatterjee (1979b)][RC79b] |
|
||
| 20 mg/天 | 26–55 岁健康男性 10 人 | 睾酮初值 482(范围 410–560)ng/dL,降至 130(110–162)ng/dL,降幅 73%(71–75%)。<br>此外 DHT 下降 51%(范围 47–55%);<br>LH 下降 39%(范围 34–45%);<br>FSH 下降 66%(范围 47–78%);<br>17-羟孕酮下降 59%;<br>雄烯二酮(A4)下降 30%;<br>磺酸睾酮(TS)下降 34%;<br>磺酸二氢睾酮(DHTS)下降 35%。<br>另有其它激素水平的数值及图表。 | [de la Torre (1979)][T79] |
|
||
| 5 或 10 mg/天 | 男性 7 人(每组 4 人);<br>有一人交替服用 5 mg/天和 10 mg/天 | 睾酮增幅为“−40%”或“–50%”。<br>5 mg/天组的睾酮初值 745 ng/dL,治疗后 460 ng/dL(–38%),停药后 668 ng/dL;<br>10 mg/天组的睾酮初值 708 ng/dL,治疗后 398 ng/dL(–44%),停药后 670 ng/dL。<br>另有 LH 及 FSH 水平数值。 | [Føgh et al. (1979)][F79];<br>[Damgaard-Pederson et al. (1980)][DP80];<br>[Føgh et al. (1980)][F80];<br>[Foegh (1983)][F83] |
|
||
| 0、5 或 10 mg/天 | 20–51 岁正常健康男性 25 人。其中:<br>5 mg/天组七人,平均年龄 37 ± 10 岁;<br>10 mg/天组八人,平均 32 ± 8 岁;<br>对照组十人,平均 32 ± 10 岁。 | 5 mg/天组中,睾酮初值 663 ± 120 ng/dL,降至 320 ± 160 ng/dL,降幅 52%;<br>10 mg/天组中,睾酮初值 692 ± 180 ng/dL,降至 340 ± 160 ng/dL,降幅 51%。<br>雌二醇水平随睾酮下降。<br>此外,5 mg/天组中,LH 初值 2.1 ± 0.7 IU/L,降至 1.4 ± 0.5 IU/L,降幅 33%;<br>10 mg/天组中,LH 初值 2.3 ± 1.0 IU/L,降至 1.2 ± 0.5 IU/L,降幅 48%。<br>5 mg/天组中,FSH 初值 3.1 ± 1.9 IU/L,降至 1.8 ± 0.9 IU/L,降幅 42%;<br>10 mg/天组中,FSH 初值 2.7 ± 1.0 IU/L,降至 1.5 ± 0.7 IU/L,降幅 44%。 | [Wang & Yeung (1980)][WY80] |
|
||
| 10 或 25 mg/天 | 29–37 岁健康男性 4 人;<br>其中 10 mg 组三人,25 mg 组一人 | 睾酮“小幅下降”。<br>雌二醇“降幅更大”。<br>LH 无明显变化。<br>FSH“在所有人中下降”,降幅“或多或少”。<br>未提供具体激素水平,但有图表。 | [Fredricsson & Carlström (1981)][FC81] |
|
||
| 10 或 20 mg/天 | 21–38 岁健康男性 30 人 | 睾酮下降 70%;<br>LH 下降 35%,而 FSH“也观测到类似降幅”。<br>未提供具体数值。 | [Moltz et al. (1982)][M82] |
|
||
| 10 mg/天 | 健康男性 5 人<br>(除 CPA 组外还有安慰剂组和<br>2、5、10 mg/天地诺孕素组,<br>每组有健康男性 5 人) | CPA 组中,睾酮初值约 600 ± 150 ng/dL,降至约 185 ng/dL,降幅 66 ± 4%。<br>另有 LH、FSH、{{< abbr "SHBG" >}} 等血清水平,以及安慰剂组、地诺孕素组的激素变化。 | [Meriggiola et al. (2002a)][M02a] |
|
||
| 10 或 20 mg/天 | 健康年轻男性 14 人(每组 7 人) | 两组中睾酮初值约 431 ng/dL,降至约 149 ng/dL,降幅 65%。<br>未提供每组单独的数值。<br>LH、FSH 抑制率在两组间无明显差异(间接表明了睾酮抑制率无差异的原因)。<br>另有使用其它孕激素制剂后引起的激素水平数值。| [Zitzmann et al. (2017)][Z17] |
|
||
|
||
</section>
|
||
|
||
以下图表摘自上述部分研究,将其结果可视化:
|
||
|
||
<section class="box">
|
||
|
||
|
||
|
||
|
||
|
||
**图 1–4:** 男性单服低剂量 CPA 期间的睾酮水平变化。资料来源:
|
||
|
||
- 上图:[Moltz et al. (1980)][M80]; [Moltz et al. (1978a)][M78a]; [Moltz et al. (1978b)][M78b]
|
||
- 中左图:[Wang & Yeung (1980)][WY80]
|
||
- 中右图:[Koch et al. (1976)][K76]; [Koch et al. (1975)][K75]
|
||
- 下图:[Meriggiola et al. (2002a)][M02a]
|
||
- 另见维基百科[图库][graph11]。
|
||
|
||
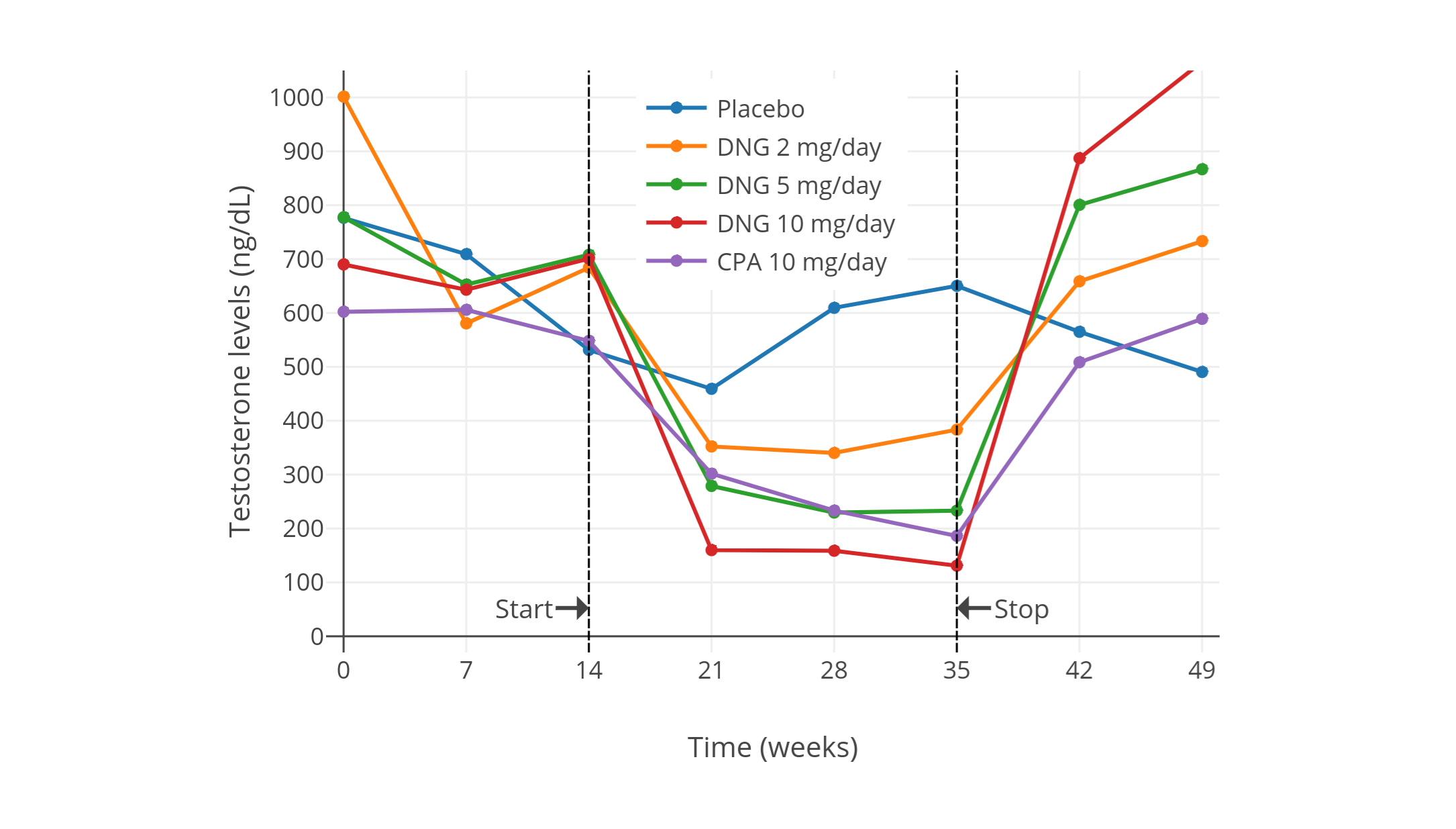
下图来自上述 2002 年的研究项目,其中睾酮水平以时间分辨荧光分析法(DELFIA)进行测定。该项目还研究了不同剂量的[地诺孕素][wiki7](DNG);其仅需 1 mg/天剂量即可抑制排卵,这点与 CPA 相似。
|
||
|
||
</section>
|
||
|
||
有关[去氧孕烯][wiki6]、[地诺孕素][wiki7]、[醋酸氯地孕酮][wiki8]等其它孕激素的研究项目,也同样发现:在男性身上可最大程度抑制睾酮水平的剂量,约为妇女抑制排卵所需剂量的 5–10 倍<sup>(维基百科 [1][wiki6-ae]; [2][wiki7-ae]; [3][wiki8-aae])</sup>。另一项有关[醋酸炔诺酮][wiki9]和[左炔诺孕酮][wiki10]的研究也印证了这点<sup>([Zitzmann et al., 2017][Z17]; [图表][graph9])</sup>。\
|
||
类似地,当用于[男性激素避孕][wiki11]时,孕激素作为抗促性腺激素制剂所需的最大有效剂量至少也有顺性别妇女所需的 5–12 倍<sup>([Foegh, 1983][F83])</sup>。\
|
||
基于后者一般使用 1 mg/天的认知,可以认为 5–10 mg/天剂量的 CPA 即可将睾酮的抑制效果最大化。该范围也与上述项目之发现相吻合。
|
||
|
||
有关更高剂量 CPA 的研究项目则发现,高剂量下睾酮的抑制效果,仅略优于低剂量的表现。近年来一些针对健康的青春期及成年女性倾向跨性别者的研究项目,发现在单服 50–100 mg/天的 CPA 的情况下,睾酮水平被抑制了 46–61%(在 4–12 个月内,从 456–602 ng/dL 降至 226–294 ng/dL)<sup>([Toorians et al., 2003][T03]; [Giltay et al., 2004][G04]; [T’Sjoen et al., 2005][TS05]; [Tack et al., 2017][T17])</sup>。\
|
||
针对老年男性前列腺癌患者的研究则发现,高剂量 CPA 单药疗法可最多将睾酮水平抑制 70–80%(降到了 50–200 ng/dL)<sup>([Gräf, Brotherton, & Neumann, 1974][GBN74]; [Jacobi et al., 1980][J80]; [图表][graph7]; [Knuth, Hano, & Nieschlag, 1984][KHN84]; [图表][graph8]; [Schröder & Radlmaier, 2002][SR02]; [Nelson, 2012][N12])</sup>。\
|
||
之所以在前列腺癌患者身上测出了更大的睾酮抑制率,可能是因为不同项目之间验血方法有所区别,以及/或者老年人群的下丘脑–垂体–性腺轴(HPG 轴)功能更弱、从而使睾酮水平更低<sup>([Liu, Takahashi, & Veldhuis, 2017][LTV17]; [Winters, Wang, & Fortigel Study Group, 2010][WWF13])</sup>。
|
||
|
||
目前已有人发现,长期使用单服孕激素疗法,会发生睾酮水平的“复原”或“逃逸”现象:尽管 _(前期)_ 其在最大有效剂量下即被显著抑制,但最终会逐渐回升。迄今该现象主要见于[醋酸甲地孕酮][wiki12]<sup>([维基百科][wiki12-aae])</sup>;但在 CPA 上亦有证实<sup>([Goldenberg & Bruchovsky, 1991][GB91]; [Saborowski, 1987][S87]; [Jacobi, Tunn, & Senge, 1982][JTS82])</sup>。其中一项研究显示,睾酮水平起初被抑制了 70% 左右;但在第 6–12 个月疗程中,其回升至 50% 左右,并在此后至多 24 个月内维持稳定。采用孕激素单药疗法抑制睾酮时,需要关注到睾酮的逃逸现象。不过,雌激素合并孕激素疗法尚未被发现与此现象有关。
|
||
|
||
## 与雌激素合用时对睾酮的抑制作用 {#testosterone-suppression-in-combination-with-estrogen}
|
||
|
||
女性倾向跨性别者一般会将 CPA 与雌激素合用。雌激素同样可抑制睾酮水平。二者合用时,可产生抑制睾酮的协同作用,其剂量相较单服雌激素或孕激素时也更少<sup>([Fink, 1979][FINK79]; [Geller & Albert, 1983][GA83]; [Bastianelli et al., 2018][B18])</sup>。 _(如果单用雌激素,)_ 要将睾酮抑制到和经外科手术(即睾丸切除术)、或经药物(即 GnRH 激动剂/拮抗剂)去势相一致的水平,则要求相对较高的雌激素水平——例如 200–500 pg/mL 之间<sup>([维基百科][wiki13-eoshl]; [图表][graph3])</sup>。由于需要超生理剂量的雌二醇方可最大程度(或接近最大程度)抑制睾酮,因此,往往采用低剂量雌二醇结合抗雄制剂/孕激素的方式来替代。
|
||
|
||
多项研究中,将雌二醇与高剂量 CPA(50–100 mg/天)合并用于女性倾向跨性别者时,其将睾酮水平抑制到了女性正常范围内(50 ng/dL 或 1.7 nmol/L 以下)<sup>([Giltay & Gooren, 2000][GG00]; [Giltay et al., 2000][G00]; [Giltay et al., 2003][G03]; [Giltay et al., 2004][G04]; [Toorians et al., 2003][T03]; [T’Sjoen et al., 2005][TS05]; [Slagter et al., 2006][S06]; [T’Sjoen et al., 2009][TS09]; [Ott et al., 2011][O11]; [Wierckx et al., 2012][W12]; [Wierckx et al., 2014][W14]; [Zubiaurre-Elorza et al., 2014][ZE14]; [Fuss et al., 2015][F15]; [Van Caenegem et al., 2015][VC15]; [Gava et al., 2016][G16]; [Bultynck et al., 2017][B17]; [Fung, Hellstern-Layefsky, & Lega, 2017][FHL17]; [Kranz et al., 2017][K17]; [Tack et al., 2017][T17]; [Wiepjes et al., 2017][W17]; [de Blok et al., 2018][DB17]; [Defreyne et al., 2018][D18]; [Vita et al., 2018][V18]; [Angus et al., 2019][A19]; [Chen et al., 2019][C19]; [Scharff et al., 2019][S19]; [van Dijk et al., 2019][VD19]; [van Velzen et al., 2019][VV19]; [Vereecke, 2019][V19]; [Vlot et al., 2019][VLOT19]; [Wiepjes et al., 2019][W19]; [Kranz, Kaufmann, & Lanzenberger, 2020][KKL20]; [Meyer et al., 2020][M20]; [Gava et al., 2020][G20]; [Sofer et al., 2020][S20]; [Vereecke et al., 2021][V21])</sup>。
|
||
|
||
1980 和 1990 年代,针对前列腺癌患者的临床研究发现,将高剂量孕激素(例如每日 100–300 mg 的 CPA、或 40–160 mg 的[醋酸甲地孕酮][wiki12])结合低剂量雌激素(例如每日 0.1–0.2 mg 的[己烯雌酚][wiki14]、或 0.5–1.5 mg 的雌二醇)使用,可完全抑制性腺的睾酮分泌,并将睾酮水平降至去势后的范围(小于 50 ng/dL)<sup>([Geller et al., 1981a][G81a]; [Geller et al., 1981b][G81b]; [Geller & Albert, 1983][GA83]; [Goldenberg et al., 1988][G88]; [Johnson et al., 1988][J88]; [Geller, 1988][GEL88]; [Venner et al., 1988][V88]; [Geller, 1991][G91-PDF]; [Goldenberg & Bruchovsky, 1991][GB91]; [Bruchovsky et al., 1993][B93]; [Goldenberg et al., 1996][G96])</sup>。与此类似,一份 1989 年的病例系列报告也披露,有 3 名女性倾向跨性别者在以 100 mg/天的 CPA 合并较低剂量雌激素治疗后,取得了同样疗效<sup>([Jequier, Bullimore, & Bishop, 1989][JBB89])</sup>。\
|
||
这里有一篇文献摘录对 CPA 用于前列腺癌治疗的情况做了详述:
|
||
|
||
> 如前所述,CPA 并未完全抑制血浆睾酮浓度:其被抑制了 70% 左右,但数值仍相当于去势后浓度的三倍。为系统研究该问题,Rennie 等 (59) 调查并对比了 12 种睾酮除去法。他们发现,将 CPA 与极低剂量的己烯雌酚(0.1 mg/天)合用,可高效去除雄激素(例如血浆睾酮、组织内的双氢睾酮)。该团队随后还发现,每日 200mg、甚至 100mg 的 CPA 足以获得相似的内分泌反馈,这也和其第二阶段临床治疗的相当正面的反馈具有关联性 (60,61)。从内分泌学视角而言,这项研究法不仅具有较多潜在优势,而且逻辑性很强:这种给药方案可结合两种制剂的抗雄效果;仅需少量雌激素,即可将血浆睾酮水平降低至经过去势的程度。一旦睾酮达到去势后水平,只需少量 CPA 即可中和剩余雄激素(主要来自肾上腺)的作用。在第三阶段疗程里,并未将合用低剂量 CPA 及己烯雌酚的疗法,与常规疗法进行比较。考虑到现有内分泌反馈与观察结果,二者合用的疗法应该具有相对其它常规疗法的竞争力。([Schröder & Radlmaier, 2002][SR02])
|
||
|
||
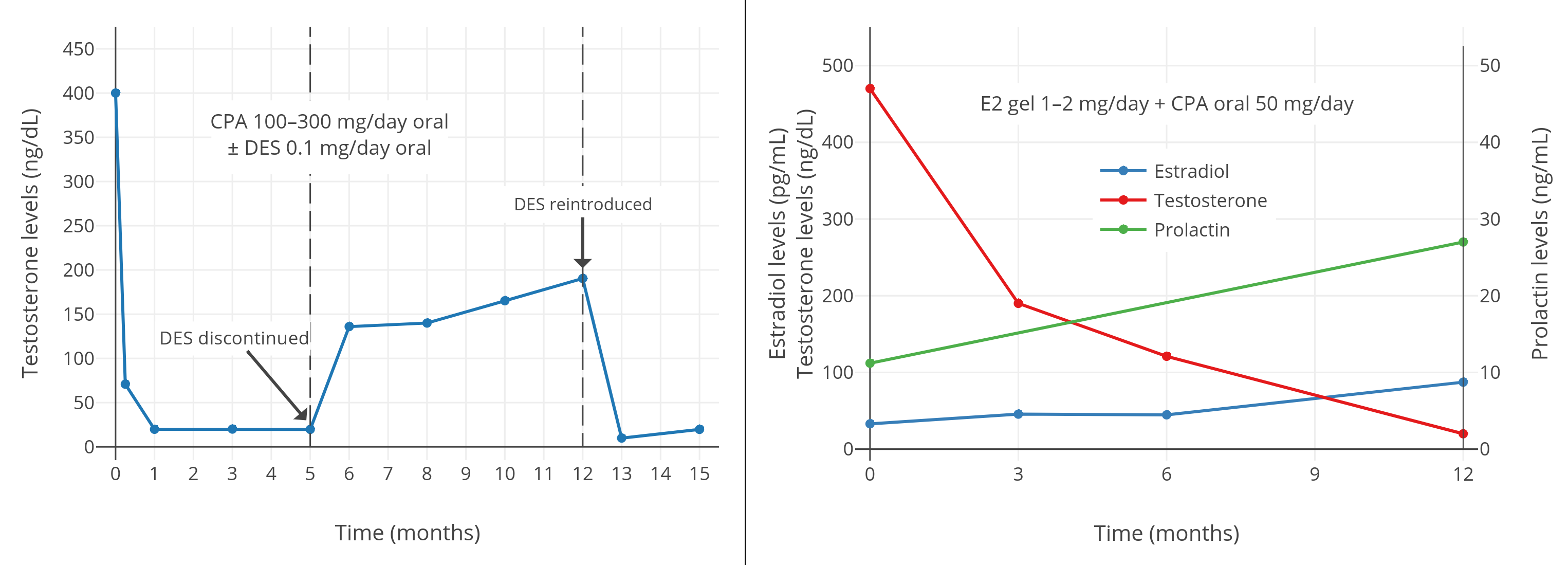
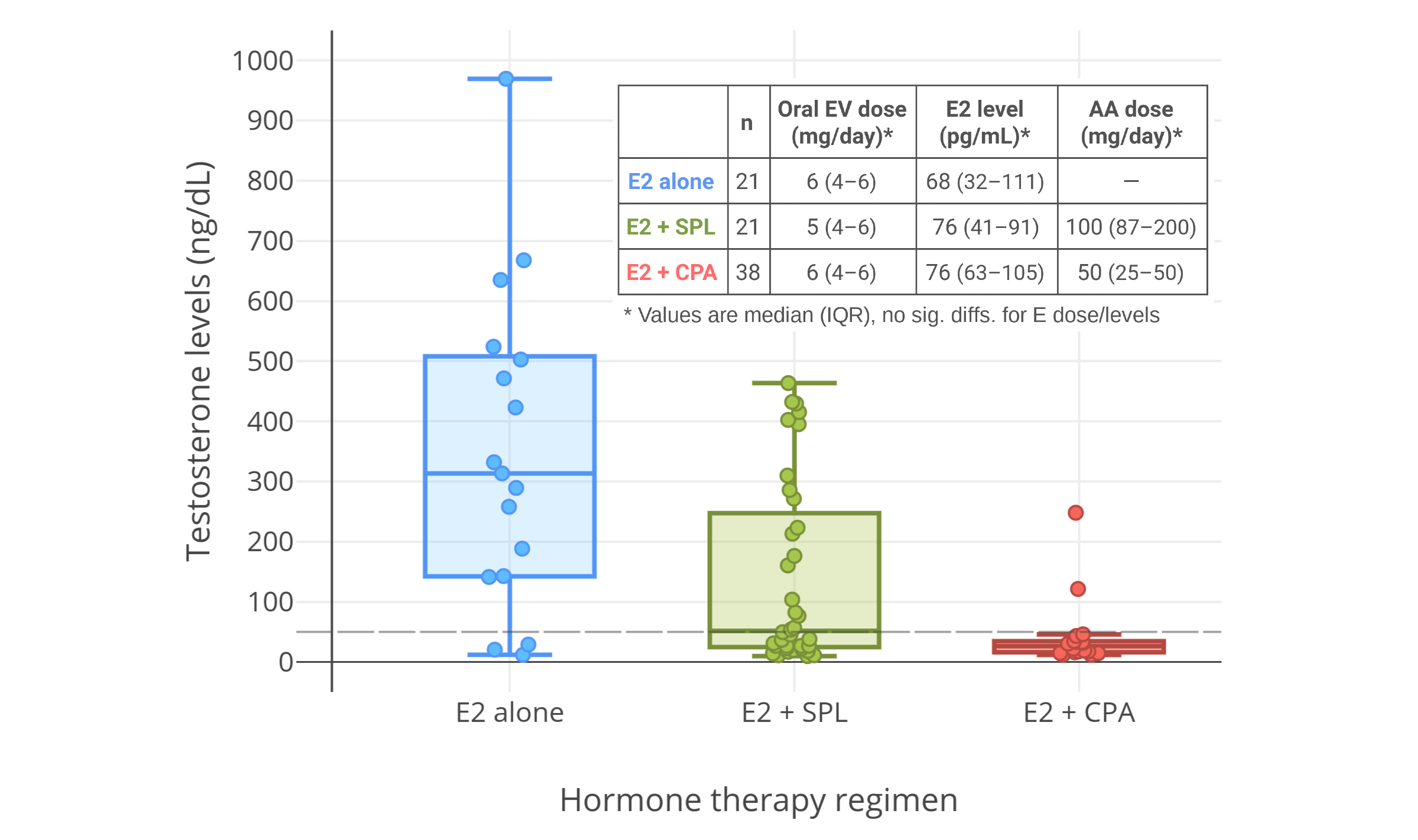
一项 2016 年的研究发现,在每日服用 50 mg 的 CPA、并涂抹 1–2 mg 雌二醇透皮凝胶的女性倾向跨性别者当中,45 pg/mL 左右的雌二醇水平(结合 CPA)并不足以将睾酮抑制到女性/去势后范围;其睾酮水平约为 120–190 ng/dL<sup>([Gava et al., 2016][G16]; [图表][graph4])</sup>。不过,85 pg/mL 左右的雌二醇水平(结合 CPA)则完全抑制了性腺的睾酮分泌,最终睾酮水平约在 20 ng/dL。因此,结合 CPA 来完全抑制睾酮所需的雌二醇浓度,需达到一个特定最小值。\
|
||
另一项 2019 年的有关 CPA 与口服戊酸雌二醇用于女性倾向跨性别者的研究显示,完全抑制睾酮水平所需的雌二醇浓度,中位数为 76 pg/mL,第 25 百分位数为 63 pg/mL<sup>([Angus et al., 2019][A19]; [图表][graph5])</sup>。
|
||
|
||
<section class="box">
|
||
|
||
|
||
|
||
|
||
**图 5–7:** 男性、女性倾向跨性别者合用 CPA 与低剂量雌激素时的睾酮浓度。资料来源:
|
||
|
||
- 左上图:[Goldenberg et al. (1988)][G88]
|
||
- 右上图:[Gava et al. (2016)][G16]
|
||
- 下图:[Angus et al. (2019)][A19]
|
||
- 另见维基百科[图库][graph11]。
|
||
|
||
右上图附注:透皮雌二醇平均剂量在第 6–12 个月有所提升,此做法可能导致了睾酮抑制率有所加强。
|
||
|
||
</section>
|
||
|
||
Fung 及其同行发现,将 25 或 50 mg/天的 CPA 结合口服雌二醇(约 3.5 mg/天)或透皮雌二醇(约 3.5 mg/天的凝胶,或约 100 μg/天的贴片)用于女性倾向跨性别者,可完全、一致地抑制性腺的睾酮分泌,其睾酮水平被抑制约 95%<sup>([Fung, Hellstern-Layefsky, & Lega, 2017][FHL17])</sup>。该剂量的雌二醇被认为可达到平均 100 pg/mL 的雌二醇水平<sup>([Aly, 2020][AW20-EED]; [维基百科][wiki15])</sup>。\
|
||
该研究恰巧比美国内分泌学会的 2017 年版《指南》<sup>([Hembree et al., 2017][H17])</sup>早发布六个月;其可能是促使后者所推荐的 CPA 剂量减少的原因(从 50–100 mg/天降至 25–50 mg/天)。
|
||
|
||
迄今罕有研究了结合低剂量 CPA 与中、低剂量雌激素对睾酮之抑制的项目。不过,基于单服 5–10 mg/天的 CPA 已可最大程度抑制睾酮的事实,可以认为其效果与高剂量的类似。根据部分研究,结合使用 5–12.5 mg/天的 CPA 与用于生理替代的睾酮的健康年轻男性,已检测不出促性腺激素(小于 0.5 IU/L),睾丸功能因此被完全抑制<sup>([Meriggiola et al., 1998][M98]; [Meriggiola et al., 2002b][M02b])</sup>。雌二醇的促性腺激素拮抗效应,相对睾酮也更强<sup>([维基百科][wiki16])</sup>,因此这些发现或许也适用于结合 CPA 与用于生理替代的雌二醇(例如平均 100–200 pg/mL 的雌二醇浓度)的情况。
|
||
|
||
依据 [Meyer 等人 (2020)][M20] 对 155 名女性倾向跨性别者合用 CPA 与雌二醇进行的研究,10、25、50 mg/天剂量的 CPA 并未在睾酮水平上形成差异;三者皆达到较高抑制率(平均降至 15–20 ng/dL,落入女性正常范围的下限)。其中所用雌二醇形态包括:口服戊酸雌二醇(剂量范围 3–10 mg/天、中位数 6 mg/天),雌二醇透皮凝胶(剂量范围 1.5–6 mg/天、中位数 2.25 mg/天),以及雌二醇透皮贴片(100 μg/天)。雌二醇浓度平均达 100 pg/mL 左右;而四分位间距(即第 25、第 75 百分位之差)则可达 100 pg/mL 左右。该研究表明,对于女性倾向跨性别者,在雌二醇水平足够高的情况下,仅需不超过 10 mg/天的 CPA 即可完全抑制睾酮水平。\
|
||
另一项研究也发现,女性倾向跨性别者使用小于 20 mg/天、和大于 50 mg/天的 CPA,在睾酮抑制率上无任何区别<sup>([Even-Zohar et al., 2020][EZ20])</sup>。
|
||
|
||
在雌二醇水平合适的情况下,为抑制睾酮甚至仅需低于 5 mg/天剂量的 CPA(例如 2 mg/天),然而尚无任何研究予以证实。不过,这种概念在对其它孕激素的研究中已有先例。例如,在一项研究里,女性倾向跨性别者每日服用 10 mg 的醋酸甲羟孕酮(该剂量大致相当于可抑制绝经前妇女排卵的 CPA 剂量:1 mg/天<sup>([表格][table1])</sup>),其睾酮水平下降了 63%,达 215 ng/dL(相比之下,雌二醇—螺内酯联合疗法可达 79 ng/dL)<sup>([Jain, Kwan, & Forcier, 2019][JKF19])</sup>。因此,可以期望极低剂量的 CPA 也能达到类似效果;而且,这种剂量还具有将人体孕激素暴露量减至正常生理水平的益处。
|
||
|
||
## 更低剂量 CPA 的临床应用 {#clinical-adoption-of-lower-doses}
|
||
|
||
考虑到高剂量 CPA 的风险、以及其低剂量下较强的睾酮抑制能力,临床上低剂量 CPA 正更多地被用于女性化激素治疗。许多文献证实了这点,其中向女性倾向跨性别者推荐了更少的剂量<sup>(例如 [Lederbogen, 2009][L09]; [Fung, Hellstern-Layefsky, & Lega, 2017][FHL17]; [Heath & Wynne, 2019][HW19]; [Winkler-Crepaz et al., 2017][WC17]; [Mahfouda et al., 2018][M19]; [Oliphant et al., 2018][O18]; [Athanasoulia-Kaspar & Stalla, 2019][AKS19]; [Bourns, 2019][B19]; [Fuss et al., 2019][F19]; [Jacobeit, 2019][J19]; [Nota, den Heijer, & Gooren, 2019][NHG19]; [澳洲与新西兰临床试验登记系统, ANZCTR, 2020][A20]; [Meyer et al., 2020][M20]; [T’Sjoen et al., 2020][TS20])</sup>。其中,Nota, den Heijer, & Gooren (2019) 的文章将 CPA 推荐的、最低必要剂量定为 10–50 mg/天,该剂量与先前过大的推荐及使用剂量(100 mg/天)<sup>([Asscheman & Gooren, 1993][AG93]; [Gooren, Giltay, & Bunck, 2008][GGB08])</sup>形成了鲜明反差。
|
||
|
||
## 更高剂量 CPA 的雄激素受体拮抗效应 {#androgen-receptor-antagonism-with-higher-doses}
|
||
|
||
CPA 的雄激素受体拮抗效应相对较弱;为达到有意义、或明显的雄激素受体拮抗效果,每日需服用 50–300 mg。但不幸的是,该剂量造成了孕激素的严重过度摄入,并与更高风险及更多不良反应有关。因此,不宜再推荐使用这种剂量。相反,CPA 应仅作为孕激素,以较低剂量来抑制睾酮水平。例如,可最大程度抑制睾酮的 CPA 剂量,大约在 10 mg/天或以下(不过 12.5 mg/天亦可接受);适宜女性倾向跨性别者的最大剂量,应接近该数值。
|
||
|
||
需要强调的是:将 CPA 结合雌激素使用,可将睾酮水平轻松抑制到女性、或去势后范围(往往还*低于* 女性平均水平),因此并不需要伴随雄激素受体拮抗作用。无论如何,如果仍需要、或期望通过雄激素受体拮抗作用来中和剩余的处在女性、或去势后水平的睾酮(例如治疗顽固性痤疮、或有其它目的),可以在 CPA 之外再补充少量非孕激素类雄激素受体拮抗剂,例如比卡鲁胺(100–200 mg/天)或螺内酯(12.5–25 mg/天);这些相对高剂量 CPA 而言更为安全。
|
||
|
||
## CPA 的推荐剂量 {#recommended-dosages}
|
||
|
||
### 抑制睾酮所需剂量 {#estrogen-plus-cyproterone-acetate}
|
||
|
||
> **如结合雌激素使用:**
|
||
|
||
对于女性倾向跨性别者,下表所示 CPA 剂量足以*最大* 程度抑制睾酮水平:
|
||
|
||
<section class="box">
|
||
|
||
**表 2:** 与雌激素并用时,可最大程度抑制女性倾向跨性别者体内睾酮的 CPA 剂量
|
||
|
||
```csv
|
||
剂型,最小剂量,最大剂量,用法
|
||
10 mg 片剂,5 mg/天,10 mg/天,每日半片到一片
|
||
50 mg 片剂,6.25 mg/天,12.5 mg/天,每日 ⅛ 到 ¼ 片
|
||
```
|
||
|
||
</section>
|
||
|
||
最初一个月内,仅使用最小剂量。一个月过后,检查睾酮水平以确认其是否处在女性、或去势后范围(50 ng/dL 以下)。如要完全抑制睾酮,需同时让雌二醇水平至少达到 65 pg/mL 左右(无关 CPA 剂量)。如果一个月后睾酮未被充分抑制、而雌二醇水平已足够,那么将 CPA 剂量加至最大推荐量,过一个月再检查睾酮水平。不过,作为替代,也可增加雌二醇用量;雌二醇水平越高,睾酮抑制率更佳。
|
||
|
||
> **如单服 CPA:**
|
||
|
||
不建议单服 CPA 以抑制睾酮;因为其存在骨质疏松风险,以及其它由性激素缺乏引起的症状<sup>([维基百科][wiki5-lhl]; [Aly, 2019][AW19-NTO])</sup>。无论如何,单服的推荐剂量基本等同于上述合用雌激素时的用量。不过,略高的剂量(10–12 mg/天)不失为一种更好的选择。
|
||
|
||
### 孕激素作用所需剂量 {#cyproterone-acetate-alone}
|
||
|
||
对于女性倾向跨性别者,下表所示 CPA 剂量基本与通常的孕激素生理暴露量(即排卵期内孕激素水平)相似:
|
||
|
||
<section class="box">
|
||
|
||
**表 3:** 可在女性倾向跨性别者体内表达生理性孕激素效力的 CPA 推荐剂量
|
||
|
||
```csv
|
||
剂型,剂量,用法
|
||
10 mg 片剂,2.5 mg/天,每日 ¼ 片
|
||
50 mg 片剂,3.125 mg/天,每日 1/16 片
|
||
```
|
||
|
||
</section>
|
||
|
||
### 达到预期剂量的手段 {#achieving-desired-dosages}
|
||
|
||
CPA 通常被制成 50 mg 片剂;这会给控制低剂量造成困难。此时可以使用切药器来切割 CPA 片剂。另外,还可每过 2 日或 3 日服用一次 CPA(而非每日服用),这样可平摊日均剂量。有一点需要补充:CPA 的清除半衰期较长,一般为 1.5–2 日,最长可达 4 日<sup>([维基百科][wiki2-m]; [图表][graph6])</sup>。因此,隔日服用、甚至每 3 日服用一次,是有市场的,也完全合情合理。
|
||
|
||
---
|
||
|
||
## 后记 {#updates}
|
||
|
||
### 后记一:正在开展的 GoLoCypro 研究 {#update-1-golocypro-study-in-progress}
|
||
|
||
澳大利亚昆士兰大学的 Judith Dean 博士,正进行一项名曰 [GoLoCypro][GLC] 的研究(2019–2022 年;[其它资料][pic1])。该项目正评估 120–350 名女性倾向跨性别者合用 CPA 与雌二醇时,不同 CPA 剂量(每周两次 12.5 mg,隔日 12.5 mg,以及每日 12.5 mg、25 mg、50 mg)所产生的影响。CPA 剂量会被滴定到一个可维持睾酮水平在 0.5–1.5 nmol/L(14–43 ng/dL)的临床目标范围内的最小值。该项目是首批有关 CPA 用于女性倾向跨性别者的[剂量范围研究][wiki17]之一;其提供的有关可充分抑制睾酮的 CPA 最小剂量的宝贵资料亦备受关注。
|
||
|
||
### 后记二:Kuijpers 等人 (2021) 与 Even Zohar 等人 (2021) 所作研究 {#update-2-kuijpers-et-al-2021-and-even-zohar-et-al-2021}
|
||
|
||
2021 年 7 月,[欧洲性别不一致调查组织网络][wiki18](ENIGI)在线发表了一篇有关女性倾向跨性别者使用低剂量 CPA 的研究论文:
|
||
|
||
- Kuijpers, S. M., Wiepjes, C. M., Conemans, E. B., Fisher, A. D., T’Sjoen, G., & den Heijer, M. (2021).
|
||
Toward a lowest effective dose of cyproterone acetate in trans women: Results from the ENIGI study.
|
||
The Journal of Clinical Endocrinology & Metabolism, 106(10), e3936–e3945. \[DOI: <https://doi.org/10.1210/clinem/dgab427>]
|
||
|
||
该项目结合使用了雌二醇与 CPA,所用剂量如下:
|
||
|
||
- **雌二醇:** 戊酸雌二醇片(每日口服 2-6 mg)、贴片(50–150 μg/天)、凝胶等;
|
||
- **CPA:** 不服用(0 mg/天)、10 mg/天、25 mg/天、50 mg/天、100 mg/天。
|
||
|
||
在不服用 CPA 的实验组,睾酮的抑制并不充分;而所有服用 CPA 的实验组皆达到完全、且一致的睾酮抑制效果。结果如下:
|
||
|
||
```csv
|
||
CPA 剂量,不服用,10 mg/天,25 mg/天,50 mg/天,100 mg/天
|
||
初始受试者人数,34,4,234,599,11
|
||
提高剂量的人数,16,1,11,2,0
|
||
降低剂量的人数,0,0,4,40,7
|
||
睾酮水平(nmol/L),5.5,0.9,0.9,1.1,0.9
|
||
睾酮水平(ng/dL),~160,~26,~26,~32,~26
|
||
检出睾酮低于 2 nmol/L 次数占比,46.3%,92.3%,96.2%,93.4%,100%
|
||
```
|
||
|
||
该项目并未提供调整 CPA 剂量之后受试者总人数,以及其验血结果。因此,10 mg/天实验组的实际人数与验血结果并不可靠。不过,基于以上“检出睾酮低于 2 nmol/L 次数占比”的数据,该实验组的验血次数应当至少有 13 次(92.3% 大致等于 12/13,但也可能是 24/26,以此类推)。论文作者考虑到该组别人数/验血次数较少,也作出了解释:
|
||
|
||
> 本项目是属于 ENIGI 倡议之下的一项前瞻性群组研究。项目中为跨性别女性所用的主要医疗方案,是每日结合使用雌激素与 50 mg 的 CPA。在实验的第一年,一些参与者使用每日 100 mg 的 CPA 剂量。此后不久,其处方调整为每日 50 mg 的 CPA。鉴于近年出现越来越多的有关 CPA 的健康担忧,CPA 剂量后来又从 50 mg 减至 25 mg、10 mg。然而,受冠状病毒 (COVID-19) 疫情影响,来自 10 mg/天剂量组的参与者的结果,只有少量可用于分析。
|
||
|
||
该项目还发现,除了睾酮抑制率之外,在泌乳素与高密度脂蛋白(胆固醇)浓度上,10 mg/天剂量 CPA 的影响要小于高剂量。该项目也测定了肝转化酶水平,但未发现不同剂量之间有差异。
|
||
|
||
论文作者总结如下:
|
||
|
||
> 总而言之,对于这组跨性别女性群体,10 mg/天的 CPA 被发现足以将睾酮浓度降低至在顺性别妇女测出的范围内。该剂量 CPA 不仅和高剂量同样有效,而且对泌乳素浓度的影响更小,也能维持更高的高密度脂蛋白(胆固醇)水平。\
|
||
> 尽管相较 CPA,选择长期副作用更少的 GnRH 激动剂会更佳;但如果 GnRH 激动剂有禁忌症、无法获取、或者无法报销的情况,那么依据本项目的发现,低剂量 CPA 也不失为一种选项。\
|
||
> 将来的研究重点,应该放在对更小剂量 CPA(例如 5 mg)之效力的评估、以及其潜在的长期副作用上。
|
||
|
||
围绕该论文的观点,论文作者之一:[Guy T’Sjoen][wiki19] 及其他同行还在一篇对女性倾向跨性别者而言最佳的激素疗法之论述中,推荐 CPA 剂量应不高于 10 或 12.5 mg/天,且不应持续服用超过两年<sup>([Glintborg et al., 2021][G21])</sup>。值得一提的是,T’Sjoen 其人被视为跨性别医学方面首屈一指的专家之一;他也是美国内分泌学会《跨性别护理指南》的联名作者之一<sup>([Hembree et al., 2017][H17])</sup>。
|
||
|
||
在 Kuijpers 及其同行发表论文后不久,以色列的 Even Zohar 及其同行也于 2021 年 7 月,发表了一篇有关女性倾向跨性别者使用低剂量 CPA 的研究论文(其最初在 2020 年 5 月,作为会议摘要发表):
|
||
|
||
- Even Zohar, N., Sofer, Y., Yaish, I., Serebro, M., Tordjman, K., & Greenman, Y. (2021).\
|
||
Low-Dose Cyproterone Acetate Treatment for Transgender Women.\
|
||
The Journal of Sexual Medicine, 18(7), 1292–1298. \[[10.1016/j.jsxm.2021.04.008][EZ21]]
|
||
- 会议摘要:[Even-Zohar et al., 2020][EZ20]
|
||
|
||
在该论文的前言部分,有如下叙述:
|
||
|
||
> 为协助医师对跨性别女性进行治疗,一些组织已发布了《治疗指南》。CPA 已被广泛用于给药方案当中。总的来说,近年来 CPA 的推荐剂量已在减小:在美国内分泌学会 2009 年版《指南》当中,CPA 推荐剂量为 50–100 mg/天;但到了 2017 年,该剂量修订为 25–50 mg/天。而在澳大利亚跨性别健康专业协会的 2019 年版《指南》所建议的 CPA 剂量,则为 12.5–25 mg/天。至于由欧洲性医学会发布的 2020 年版《指南》,则将 CPA 剂量修订为 10–50 mg/天。迄今尚无有关不同 CPA 剂量的效力与安全性之间的对比数据的论文发表。
|
||
|
||
该论文的研究者发现,当合用雌二醇时,低剂量 CPA(10–20 mg/天)的睾酮抑制率与高剂量(50–100 mg/天)一致。睾酮水平降低到了(或接近)女性/去势后范围(一般不超过 2 nmol/L 或 58 pg/mL)。\
|
||
有 38 名女性倾向跨性别者服用低剂量 CPA,其中 32 名(84%)的剂量为 10 mg/天、6 名(16%)的剂量为 20 mg/天,平均剂量 11.6 ± 3.7 mg/天。雌二醇则通过透皮贴片(平均剂量 83.7 ± 36.5 μg/天)、透皮凝胶(平均剂量 3.8 ± 1.2 g/天)或者口服(平均剂量 4.1 ± 1.7 mg/天)的形式给药。后续在低剂量、高剂量 CPA 组别当中,雌二醇浓度平均达到了约 110 到 350 pmol/L(或约 30–95 pg/mL)。\
|
||
除睾酮抑制效果之外,研究还发现了:在 12 个月激素治疗过后,低剂量 CPA 组别的泌乳素水平(398 ± 69 mIU/mL)显著低于高剂量的水平(804 ± 121 mIU/mL)。
|
||
|
||
基于这些发现,论文作者叙述如下:
|
||
|
||
> 我们建议,现有的《临床实践指南》应修订其为跨性别女性推荐的 CPA 剂量。
|
||
|
||
[Kuijper 等人 (2021)][K21] 和 [Even Zohar 等人 (2021)][EZ21] 均声明,其为首次揭露女性倾向跨性别者服用低剂量 CPA 之疗效。不过,这项成就其实应该属于 [Meyer 等人 (2020)][M20];其于 2020 年 2 月便已发现,女性倾向跨性别者合用雌二醇与 10、25、50 mg/天的 CPA 时,可获得一致的睾酮抑制效果。
|
||
|
||
[Lim 等人 (2020)][L20] 在 2020 年 5/7 月发表了一篇论文;其研究课题并非与 CPA 及睾酮抑制效果有关,不过,其报告了 26 名女性倾向跨性别者分别使用口服与透皮雌二醇时,睾酮水平中位数(四分位间距)分别为 0.6 (0.4–1.0) nmol/L 和 0.9 (0.7–1.6) nmol/L;她们还分为了合用雌二醇与低剂量 CPA(12.5–18.8 mg/天,中位数 12.5;共 14 人)、摘除性腺后单用雌二醇(共 9 人)、以及合用雌二醇与螺内酯(共 3 人)等组别。
|
||
|
||
### 后记三:Kumar 等人 (2021) 所报告的与低剂量 CPA 有关的肝衰竭病例 {#update-3-kumar-et-al-2021-lower-dose-cpa-liver-failure-case}
|
||
|
||
一篇于 2021 年 12 月发表的论文报告了一起由低剂量 CPA 引起的致命[肝衰竭][wiki20]病例:
|
||
|
||
- Kumar, P., Reddy, S., Kulkarni, A., Sharma, M., & Rao, P. N. (2021).\
|
||
Cyproterone acetate induced Acute liver failure: Case report and review of the literature.\
|
||
Journal of Clinical and Experimental Hepatology, 11(6), 739–741. \[DOI: [10.1016/j.jceh.2021.01.003][KUMAR21]]
|
||
|
||
该病例描述了一名 30 岁顺性别妇女连续六个月服用 25 mg/天的 CPA 以治疗[多毛症][wiki21],但患上了[急性肝衰竭][wiki22];住院四天后不治身亡。这是迄今报告的第二起和低于 100 mg/天剂量的 CPA 之[肝毒性][wiki23]有关的病例<sup>([维基百科][wiki5-lt]; [表格][table4])</sup>,也是第一起有关 CPA 对于顺性别妇女之肝毒性的病例。该病例表明,在肝毒性上,即便相对较低的 CPA 剂量:25 mg/天,也并非足够安全。\
|
||
该论文还强调了女性倾向跨性别者应使用最低有效剂量(不大于 10–12.5 mg/天)的重要性。
|
||
|
||
<!-- 以下为 2022 年 3 月以来的增补内容 -->
|
||
|
||
### 后记四:Coleman 等人 (2022) 所作 WPATH SOC8《指南》 {#update-4-coleman-et-al-2022-wpath-soc8-guidelines}
|
||
|
||
2022 年 9 月,[世界跨性别人士健康专业协会][wiki24](WPATH)发布了第八版《[跨性别及性别多元化人群健康护理标准][wiki25]》(SOC8),其中首次为跨性别激素治疗提供建议<sup>([Coleman et al., 2022][C22])</sup>。其向女性倾向跨性别者推荐的 CPA 剂量为 10 mg/天<sup>([Coleman et al., 2022][C22])</sup>。此剂量远比早前发布的《跨性别护理指南》所建议的要低;这也是首次有主流《指南》推荐如此低的剂量。\
|
||
该《标准》提及了 [Kuijpers 等人 (2021)][K21] 并支持其推荐的剂量[但并未提及 [Even Zohar 等人 (2021)][EZ21] 或 [Meyer 等人 (2020)][M20]],同时还讨论了脑膜瘤和高泌乳素水平等与 CPA 剂量有关的风险<sup>([Coleman et al., 2022][C22])</sup>。\
|
||
考虑到 WPATH 的这篇《标准》在跨性别健康领域的重要地位和影响力,可以认为,全球范围内在女性化激素治疗当中使用低剂量 CPA 的情况将会更普遍。有当下公认的循证实践在先,不应再继续使用高剂量 CPA。
|
||
|
||
### 后记五:Collet 等人 (2023) 所作研究 {#update-5-collet-et-al-2023}
|
||
|
||
2022 年 10 月,一项对雌二醇与 CPA 的睾酮抑制效果进行细致评估的研究,公开发表了论文:
|
||
|
||
- Collet, S., Gieles, N., Wiepjes, C. M., Heijboer, A. C., Reyns, T., Fiers, T., Lapauw, B., den Heijer, M., & T’Sjoen, G. (2023).\
|
||
Changes in serum testosterone and adrenal androgen levels in transgender women with and without gonadectomy.\
|
||
*The Journal of Clinical Endocrinology & Metabolism*, *108*(2), 331–338. \[DOI:[10.1210/clinem/dgac576][C23]]
|
||
|
||
该项目属于[欧洲性别不一致调查组织网络][wiki18](ENIGI)倡议之下的一部分,其中大多数患者来自荷兰阿姆斯特丹的诊所、以及比利时根特的诊所。
|
||
|
||
该项目中有 275 名女性倾向跨性别者接受雌二醇合并 CPA 治疗。她们的总睾酮、游离睾酮水平,以及[由肾上腺分泌的雄激素][wiki26]——[脱氢表雄酮][wiki27](DHEA)、[硫酸脱氢表雄酮][wiki28](DHEA-S)与[雄烯二酮][wiki29](A4)等——之水平,以[液相色谱–质谱联用法][wiki30](LC-MS)进行测定;测定时间定于跟踪开始时(基线)、第 3 个月、第 12 个月、第 2–4 年以及经手术切除性腺之后(此时不再服用 CPA)。至于其雌二醇水平,则以 LC-MS(阿姆斯特丹方面)或免疫测定法(根特方面)进行测定。\
|
||
其所用雌二醇形式与剂量,大多为 4 mg/天的口服戊酸雌二醇或 100 μg/天的雌二醇透皮贴片;而 CPA 的剂量多为 25–50 mg/天。\
|
||
这些人当中有大约一半,在以激素治疗约两年之后都接受了性腺切除术。
|
||
|
||
结果显示:
|
||
|
||
- 在不同跟踪时长下,以 LC-MS 和免疫测定法测定的雌二醇水平中位数,分别为 49–75 pg/mL(合 180–275 pmol/L)和 63–69 pg/mL(232–255 pmol/L)。
|
||
- 在接受激素治疗达三个月之后,总睾酮水平从 536 ng/dL (18.6 nmol/L) 降至 12 ng/dL (0.40 nmol/L),降幅 97.1%;而游离睾酮水平从 109 pg/mL (378 pmol/L) 降至 2.0 pg/mL (7.1 pmol/L),降幅 98.3%;此后,总睾酮、游离睾酮水平维持稳定。
|
||
- 至于 DHEA、DHEA-S 及 A4 等激素的水平,降幅则分别达 24.9–28.0%、20.1–23.5% 及 36.5%,到了激素治疗第 3–12 月之后也基本不变。
|
||
- 接受性腺切除术并停用 CPA 之后,并未出现睾酮水平的变化。
|
||
|
||
论文作者指出,在本研究中,接受激素治疗的女性倾向跨性别者的睾酮水平,与顺性别妇女接近或稍低于之。
|
||
|
||
## 参考文献 {#references}
|
||
|
||
- Angus, L., Leemaqz, S., Ooi, O., Cundill, P., Silberstein, N., Locke, P., Zajac, J. D., & Cheung, A. S. (2019). Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. *Endocrine Connections*, *8*(7), 935–940. \[DOI:[10.1530/ec-19-0272][A19]]
|
||
- Asscheman, H., & Gooren, L. J. (1992). Hormone Treatment in Transsexuals. In Bocking, W. O., Coleman, E. (Eds). *Gender Dysphoria: Interdisciplinary Approaches in Clinical Management* (pp. 39–54). Binghamton: Haworth Press. / *Journal of Psychology & Human Sexuality*, *5*(4), 39–54. \[[Google 学术][AG93-GS]] \[[Google 阅读][AG93-GB]] \[DOI:[10.1300/J056v05n04\_03][AG93]]
|
||
- Athanasoulia-Kaspar, A. P., & Stalla, G. K. (2019). Endokrinologische Betreuung von Patienten mit Transsexualität. *Geburtshilfe und Frauenheilkunde*, *79*(7), 672–675. \[DOI:[10.1055/a-0801-3319][AKS19]]
|
||
- Bastianelli, C., Farris, M., Rosato, E., Brosens, I., & Benagiano, G. (2018). Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation. *Expert Review of Clinical Pharmacology*, *11*(11), 1085–1098. \[DOI:[10.1080/17512433.2018.1536544][B18]]
|
||
- Bourns, A. (2019). *Guidelines for Gender-Affirming Primary Care with Trans and Non-Binary Patients, 4th Edition.* Toronto: Rainbow Health Ontario/Sherbourne Health. \[[URL][B19]] \[[PDF][B19-PDF]]
|
||
- Bruchovsky, N., Larry Goldenberg, S., Akakura, K., & Rennie, P. S. (1993). Luteinizing hormone-releasing hormone agonists in prostate cancer. Elimination of flare reaction by pretreatment with cyproterone acetate and low-dose diethylstilbestrol. *Cancer*, *72*(5), 1685–1691. \[DOI:[10.1002/1097-0142(19930901)72:5<1685::aid-cncr2820720532>3.0.co;2-3][B93]]
|
||
- Bultynck, C., Pas, C., Defreyne, J., Cosyns, M., den Heijer, M., & T’Sjoen, G. (2017). Self-perception of voice in transgender persons during cross-sex hormone therapy. *The Laryngoscope*, *127*(12), 2796–2804. \[DOI:[10.1002/lary.26716][B17]]
|
||
- Chen, H., Wiepjes, C. M., van Schoor, N. M., Heijboer, A. C., de Jongh, R. T., den Heijer, M., & Lips, P. (2019). Changes of Vitamin D-Binding Protein, and Total, Bioavailable, and Free 25-Hydroxyvitamin D in Transgender People. *The Journal of Clinical Endocrinology & Metabolism*, *104*(7), 2728–2734. \[DOI:[10.1210/jc.2018-02602][C19]]
|
||
- Coleman, E., Radix, A. E., Bouman, W. P., Brown, G. R., de Vries, A. L., Deutsch, M. B., Ettner, R., Fraser, L., Goodman, M., Green, J., Hancock, A. B., Johnson, T. W., Karasic, D. H., Knudson, G. A., Leibowitz, S. F., Meyer-Bahlburg, H. F., Monstrey, S. J., Motmans, J., Nahata, L., … & Arcelus, J. (2022). \[World Professional Association for Transgender Health (WPATH)] Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. *International Journal of Transgender Health*, *23*(Suppl 1), S1–S259. \[DOI:[10.1080/26895269.2022.2100644][C22]] \[[URL][C22-URL]] \[[PDF][C22-PDF]]
|
||
- Collet, S., Gieles, N., Wiepjes, C. M., Heijboer, A. C., Reyns, T., Fiers, T., Lapauw, B., den Heijer, M., & T’Sjoen, G. (2023). Changes in serum testosterone and adrenal androgen levels in transgender women with and without gonadectomy. *The Journal of Clinical Endocrinology & Metabolism*, *108*(2), 331–338. \[DOI:[10.1210/clinem/dgac576][C23]]
|
||
- Damgaard-Pedersen, F., & Føgh, M. (1980). The effect of cyproterone acetate on serum lipids in normal men. *Acta Endocrinologica*, *94*(2), 280–283. \[DOI:[10.1530/acta.0.0940280][DP80]]
|
||
- de Blok, C. J., Klaver, M., Wiepjes, C. M., Nota, N. M., Heijboer, A. C., Fisher, A. D., Schreiner, T., T’Sjoen, G., & den Heijer, M. (2017). Breast Development in Transwomen After 1 Year of Cross-Sex Hormone Therapy: Results of a Prospective Multicenter Study. *The Journal of Clinical Endocrinology & Metabolism*, *103*(2), 532–538. \[DOI:[10.1210/jc.2017-01927][DB17]]
|
||
- Defreyne, J., Vantomme, B., Van Caenegem, E., Wierckx, K., De Blok, C., Klaver, M., Nota, N. M., Van Dijk, D., Wiepjes, C. M., Den Heijer, M., & T’Sjoen, G. (2018). Prospective evaluation of hematocrit in gender-affirming hormone treatment: results from European Network for the Investigation of Gender Incongruence. *Andrology*, *6*(3), 446–454. \[DOI:[10.1111/andr.12485][D18]]
|
||
- Endrikat, J., Gerlinger, C., Richard, S., Rosenbaum, P., & Düsterberg, B. (2011). Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide. *Contraception*, *84*(6), 549–557. \[DOI:[10.1016/j.contraception.2011.04.009][E11]]
|
||
- Even-Zohar, N., Sofer, Y., Yaish, I., Serebro, M., Tordjman, K., & Greenman, Y. (2020). SUN-042 Low Dose Cyproterone Acetate for the Treatment of Transgender Women - a Retrospective Study. *Journal of the Endocrine Society*, *4*(Suppl 1), A715–A715. \[DOI:[10.1210/jendso/bvaa046.1412][EZ20]]
|
||
- Even Zohar, N., Sofer, Y., Yaish, I., Serebro, M., Tordjman, K., & Greenman, Y. (2021). Low-Dose Cyproterone Acetate Treatment for Transgender Women. *The Journal of Sexual Medicine*, *18*(7), 1292–1298. \[DOI:[10.1016/j.jsxm.2021.04.008][EZ21]]
|
||
- Fink, G. (1979). Feedback Actions of Target Hormones on Hypothalamus and Pituitary With Special Reference to Gonadal Steroids. *Annual Review of Physiology*, *41*(1), 571–585. \[DOI:[10.1146/annurev.ph.41.030179.003035][FINK79]]
|
||
- Føgh, M., Corker, C. S., Hunter, W. M., McLean, H., Philip, J., Schou, G., & Shakkebæk, N. E. (1979). The effects of low doses of cyproterone acetate on some functions of the reproductive system in normal men. *Acta Endocrinologica*, *91*(3), 545–552. \[DOI:[10.1530/acta.0.0910545][F79]]
|
||
- Føgh, M., Knudsen, J. B., & Gormsen, J. (1980). Effect of cyproterone acetate on platelet aggregability, fibrinolytic activity and fibrinolytic capacity in normal men. *Acta Endocrinologica*, *94*(3), 430–432. \[DOI:[10.1530/acta.0.0940430][F80]]
|
||
- Foegh, M. (1983). Evaluation of Steroids as COntraceptives in Men. *Acta Endocrinologica*, *104*(3 Suppl b), S9–S48. \[DOI:[10.1530/acta.0.104s009][F83]]
|
||
- Fredricsson, B., & Carlström, K. (1981). Effects of Low Doses of Cyproterone Acetate on Sperm Morphology and some other Parameters of Reproduction in Normal Men. *Andrologia*, *13*(4), 369–375. \[DOI:[10.1111/j.1439-0272.1981.tb00067.x][FC81]]
|
||
- Fung, R., Hellstern-Layefsky, M., & Lega, I. (2017). Is a lower dose of cyproterone acetate as effective at testosterone suppression in transgender women as higher doses? *International Journal of Transgenderism*, *18*(2), 123–128. \[DOI:[10.1080/15532739.2017.1290566][FHL17]]
|
||
- Fuss, J., Hellweg, R., Van Caenegem, E., Briken, P., Stalla, G. K., T’Sjoen, G., & Auer, M. K. (2015). Cross-sex hormone treatment in male-to-female transsexual persons reduces serum brain-derived neurotrophic factor (BDNF). *European Neuropsychopharmacology*, *25*(1), 95–99. \[DOI:[10.1016/j.euroneuro.2014.11.019][F15]]
|
||
- Fuss, J., Claro, L., Ising, M., Biedermann, S. V., Wiedemann, K., Stalla, G. K., Briken, P., & Auer, M. K. (2019). Does sex hormone treatment reverse the sex-dependent stress regulation? A longitudinal study on hypothalamus-pituitary-adrenal (HPA) axis activity in transgender individuals. *Psychoneuroendocrinology*, *104*, 228–237. \[DOI:[10.1016/j.psyneuen.2019.02.023][F19]]
|
||
- Gava, G., Cerpolini, S., Martelli, V., Battista, G., Seracchioli, R., & Meriggiola, M. C. (2016). Cyproterone acetate\_vs\_leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness. *Clinical Endocrinology*, *85*(2), 239–246. \[DOI:[10.1111/cen.13050][G16]]
|
||
- Gava, G., Mancini, I., Alvisi, S., Seracchioli, R., & Meriggiola, M. C. (2020). A comparison of 5-year administration of cyproterone acetate or leuprolide acetate in combination with estradiol in transwomen. *European Journal of Endocrinology*, *183*(6), 561–569. \[DOI:[10.1530/eje-20-0370][G20]]
|
||
- Geller, J., Albert, J., Yen, S. S., Geller, S., & Loza, D. (1981). Medical Castration of Males with Megestrol Acetate and Small Doses of Diethylstilbestrol\*. *The Journal of Clinical Endocrinology & Metabolism*, *52*(3), 576–580. \[DOI:[10.1210/jcem-52-3-576][G81a]]
|
||
- Geller, J., Albert, J., Yen, S. S., Geller, S., & Loza, D. (1981). Medical castration with megestrol acetate and minidose of diethylstilbestrol. *Urology*, *17*(4 Suppl), 27–33. \[[Google 学术][G81b-GS]] \[[PubMed][G81b]]
|
||
- Geller, J., & Albert, J. D. (1983). Comparison of various hormonal therapies for prostatic carcinoma. *Seminars in Oncology*, *10*(4 Suppl 4), 34–41. \[[Google 学术][GA83-GS]] \[[PubMed][GA83-PM]] \[[PDF][GA83]]
|
||
- Geller, J. (1988). Megestrol acetate and minidose estrogen in prostatic carcinoma. *Urology*, *32*(3), 281–282. \[DOI:[10.1016/0090-4295(88)90402-5][GEL88]]
|
||
- Geller J. (1991). Megestrol acetate plus low-dose estrogen in the management of advanced prostatic carcinoma. *The Urologic Clinics of North America*, *18*(1), 83–91. \[DOI:[10.1016/S0094-0143(21)01395-1][G91]] \[[PDF][G91-PDF]]
|
||
- Giltay, E. J., & Gooren, L. J. (2000). Effects of Sex Steroid Deprivation/Administration on Hair Growth and Skin Sebum Production in Transsexual Males and Females. *The Journal of Clinical Endocrinology & Metabolism*, *85*(8), 2913–2921. \[DOI:[10.1210/jcem.85.8.6710][GG00]]
|
||
- Giltay, E. J., Gooren, L. J., Emeis, J. J., Kooistra, T., & Stehouwer, C. D. (2000). Oral, but Not Transdermal, Administration of Estrogens Lowers Tissue-Type Plasminogen Activator Levels in Humans Without Affecting Endothelial Synthesis. *Arteriosclerosis, Thrombosis, and Vascular Biology*, *20*(5), 1396–1403. \[DOI:[10.1161/01.atv.20.5.1396][G00]]
|
||
- Giltay, E. J., Verhoef, P., Gooren, L. J., Geleijnse, J. M., Schouten, E. G., & Stehouwer, C. D. (2003). Oral and transdermal estrogens both lower plasma total homocysteine in male-to-female transsexuals. *Atherosclerosis*, *168*(1), 139–146. \[DOI:[10.1016/s0021-9150(03)00090-x][G03]]
|
||
- Giltay, E. J., Gooren, L. J., Toorians, A. W., Katan, M. B., & Zock, P. L. (2004). Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. *The American Journal of Clinical Nutrition*, *80*(5), 1167–1174. \[DOI:[10.1093/ajcn/80.5.1167][G04]]
|
||
- Glintborg, D., T’Sjoen, G., Ravn, P., & Andersen, M. S. (2021). MANAGEMENT OF ENDOCRINE DISEASE: Optimal feminizing hormone treatment in transgender people. *European Journal of Endocrinology*, *185*(2), R49–R63. \[DOI:[10.1530/eje-21-0059][G21]]
|
||
- Goldenberg, S. L., Bruchovsky, N., Rennie, P. S., & Coppin, C. M. (1988). The Combination of Cyproterone Acetate and Low Dose Diethylstilbestrol in the Treatment of Advanced Prostatic Carcinoma. *Journal of Urology*, *140*(6), 1460–1465. \[DOI:[10.1016/s0022-5347(17)42073-8][G88]]
|
||
- Goldenberg, S. L., & Bruchovsky, N. (1991). Use of cyproterone acetate in prostate cancer. *The Urologic Clinics of North America*, *18*(1), 111–122. \[DOI:[10.1016/S0094-0143(21)01398-7][GB91]] \[[PDF][GB91-PDF]]
|
||
- Goldenberg, S., Bruchovsky, N., Gleave, M., & Sullivan, L. (1996). Low-dose cyproterone acetate plus mini-dose diethylstilbestrol—A protocol for reversible medical castration. *Urology*, *47*(6), 882–884. \[DOI:[10.1016/s0090-4295(96)00048-9][G96]]
|
||
- Gooren, L. J., Giltay, E. J., & Bunck, M. C. (2008). Long-Term Treatment of Transsexuals with Cross-Sex Hormones: Extensive Personal Experience. *The Journal of Clinical Endocrinology & Metabolism*, *93*(1), 19–25. \[DOI:[10.1210/jc.2007-1809][GGB08]]
|
||
- Gräf, K., Brotherton, J., & Neumann, F. (1974). Clinical Uses of Antiandrogens. In Hughes, A., Hasan, S. H., Oertel, G. W., Voss, H. E., Bahner, F., Neumann, F., Steinbeck, H., Gräf, K.-J., Brotherton, J., Horn, H. J., & Wagner, R. K. (Eds.). *Androgens II and Antiandrogens / Androgene II und Antiandrogene* (*Handbuch der experimentellen Pharmakologie/Handbook of Experimental Pharmacology, Volume 35, Part 2*) (pp. 485–542). Berlin/Heidelberg: Springer. \[DOI:[10.1007/978-3-642-80859-3\_7][GBN74]]
|
||
- Hammerstein, J., Meckies, J., Leo-Rossberg, I., Moltz, L., & Zielske, F. (1975). Use of cyproterone acetate (CPA) in the treatment of acne, hirsutism and virilism. *Journal of Steroid Biochemistry*, *6*(6), 827–836. \[DOI:[10.1016/0022-4731(75)90311-8][H75]]
|
||
- Hammerstein, J. (1979). Cyproterone Acetate. In Jacobs, H. S. (Ed.). *Advances in Gynaecological Endocrinology: Proceedings of the Sixth Study Group of the Royal College of Obstetricians and Gynaecologists, 18th and 19th October, 1978* (pp. 367–382). London: The College. \[[Google 学术][H79-GS]] \[[Google 阅读][H79-GB]] \[[PDF][H79]]
|
||
- Hammerstein, J. (1990). Antiandrogens: Clinical Aspects. In Orfanos, C. E., & Happle, R. (Eds.). *Hair and Hair Diseases* (pp. 827–886). Berlin/Heidelberg: Springer. \[DOI:[10.1007/978-3-642-74612-3\_35][H90]]
|
||
- Heath, R. A., & Wynne, K. (2019). *A Guide to Transgender Health: State-of-the-art Information for Gender-Affirming People and Their Supporters* (p. 122). Santa Barbara: Praeger/ABC-CLIO. \[[Google 阅读][HW19]]
|
||
- Hembree, W. C., Cohen-Kettenis, P., Delemarre-Van De Waal, H. A., Gooren, L. J., Meyer III, W. J., Spack, N. P., Tangpricha, V., & Montori, V. M. (2009). Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. *The Journal of Clinical Endocrinology & Metabolism*, *94*(9), 3132–3154. \[DOI:[10.1210/jc.2009-0345][H09]]
|
||
- Hembree, W. C., Cohen-Kettenis, P. T., Gooren, L., Hannema, S. E., Meyer, W. J., Murad, M. H., Rosenthal, S. M., Safer, J. D., Tangpricha, V., & T’Sjoen, G. G. (2017). Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society\* Clinical Practice Guideline \[2nd Version]. *The Journal of Clinical Endocrinology & Metabolism*, *102*(11), 3869–3903. \[DOI:[10.1210/jc.2017-01658][H17]] \[[PDF][H17-PDF]]
|
||
- Jacobeit, J. W. (2019). Die hormonelle Behandlung von adulten Trans\*Personen (in Deutschland). \[Hormonal treatment of adult trans\* persons (in Germany).] *Journal für Klinische Endokrinologie und Stoffwechsel*, *12*(3), 102–110. \[DOI:[10.1007/s41969-019-00080-x][J19]]
|
||
- Jacobi, G. H., Altwein, J. E., Kurth, K. H., Basting, R., & Hohenfellner, R. (1980). Treatment of Advanced Prostatic Cancer with Parenteral Cyproterone Acetate: A Phase III Randomised Trial\*. *British Journal of Urology*, *52*(3), 208–215. \[DOI:[10.1111/j.1464-410x.1980.tb02961.x][J80]]
|
||
- Jacobi, G. H., Tunn, U., & Senge, T. (1982). Clinical experience with cyproterone acetate for palliation of inoperable prostate cancer. In Jacobi, G. H., & Hohenfellner, R. (Eds.). *Prostate Cancer*, *3*, 305–319. Baltimore: Williams & Wilkins. \[[Google 学术][JTS82-GS]] \[[PDF][JTS82]]
|
||
- Jain, J., Kwan, D., & Forcier, M. (2019). Medroxyprogesterone Acetate in Gender-Affirming Therapy for Transwomen: Results From a Retrospective Study. *The Journal of Clinical Endocrinology & Metabolism*, *104*(11), 5148–5156. \[DOI:[10.1210/jc.2018-02253][JKF19]]
|
||
- Jequier, A. M., Bullimore, N. J., & Bishop, M. J. (1989). Cyproterone Acetate and a Small Dose of Oestrogen in the Pre-operative Management of Male Transsexuals. A Report of Three Cases. \[Cyproteronacetat und kleine Östrogendosis in dem präoperativen Management männlicher Transsexueller. Bericht über drei Fälle.] *Andrologia*, *21*(5), 456–461. \[DOI:[10.1111/j.1439-0272.1989.tb02447.x][JBB89]]
|
||
- Johnson, D. E., Babaian, R. J., Swanson, D. A., Von Eschenbach, A. C., Wishnow, K. I., & Tenney, D. (1988). Medical castration using megestrol acetate and minidose estrogen. *Urology*, *31*(5), 371–374. \[DOI:[10.1016/0090-4295(88)90726-1][J88]]
|
||
- Knuth, U. A., Hano, R., & Nieschlag, E. (1984). Effect of Flutamide or Cyproterone Acetate on Pituitary and Testicular Hormones in Normal Men. *The Journal of Clinical Endocrinology & Metabolism*, *59*(5), 963–969. \[DOI:[10.1210/jcem-59-5-963][KHN84]]
|
||
- Koch, U. J., Lorenz, F., Danehl, K., & Hammerstein, J. (1975). Über die Verwendbarkeit von Cyproteronacetat zur Fertilitätshemmung beim Mann. Morphologische Veränderungen und Einflüsse auf die Spermienmotilität. *Archiv für Gynäkologie*, *219*(1–4), 581–582. \[DOI:[10.1007/bf00669258][K75]]
|
||
- Koch, U., Lorenz, F., Danehl, K., Ericsson, R., Hasan, S., Keyserlingk, D., Lübke, K., Mehring, M., Römmler, A., Schwartz, U., & Hammerstein, J. (1976). Continuous oral low-dosage cyproterone acetate for fertility regulation in the male? A trend analysis in 15 volunteers. *Contraception*, *14*(2), 117–135. \[DOI:[10.1016/0010-7824(76)90081-0][K76]]
|
||
- Kranz, G. S., Seiger, R., Kaufmann, U., Hummer, A., Hahn, A., Ganger, S., Tik, M., Windischberger, C., Kasper, S., & Lanzenberger, R. (2017). Effects of sex hormone treatment on white matter microstructure in individuals with gender dysphoria. *NeuroImage*, *150*, 60–67. \[DOI:[10.1016/j.neuroimage.2017.02.027][K17]]
|
||
- Kranz, G. S., Kaufmann, U., & Lanzenberger, R. (2020). Probing the Impact of Gender-Affirming Hormone Treatment on Odor Perception. *Chemical Senses*, *45*(1), 37–44. \[DOI:[10.1093/chemse/bjz069][KKL20]]
|
||
- Kuijpers, S. M., Wiepjes, C. M., Conemans, E. B., Fisher, A. D., T’Sjoen, G., & den Heijer, M. (2021). Toward a Lowest Effective Dose of Cyproterone Acetate in Trans Women: Results From the ENIGI Study. *The Journal of Clinical Endocrinology & Metabolism*, *106*(10), e3936–e3945. \[DOI:[10.1210/clinem/dgab427][K21]]
|
||
- Kumar, P., Reddy, S., Kulkarni, A., Sharma, M., & Rao, P. N. (2021). Cyproterone acetate induced acute liver failure: case report and review of the literature. *Journal of Clinical and Experimental Hepatology*, *11*(6), 739–741. \[DOI:[10.1016/j.jceh.2021.01.003][KUMAR21]]
|
||
- Lederbogen, S. (2009). Hormonbehandlung. *PiD - Psychotherapie im Dialog*, *10*(1), 41–43. \[DOI:[10.1055/s-0028-1090190][L09]]
|
||
- Lim, H. Y., Leemaqz, S. Y., Torkamani, N., Grossmann, M., Zajac, J. D., Nandurkar, H., Ho, P., & Cheung, A. S. (2020). Global Coagulation Assays in Transgender Women on Oral and Transdermal Estradiol Therapy. *The Journal of Clinical Endocrinology & Metabolism*, *105*(7), e2369–e2377. \[DOI:[10.1210/clinem/dgaa262][L20]]
|
||
- Liu, P. Y., Takahashi, P., & Veldhuis, J. D. (2017). An Ensemble Perspective of Aging-Related Hypoandrogenemia in Men. In Winters, S. J., & Huhtaniemi, I. T. (Eds.). *Male Hypogonadism: Basic, Clinical and Therapeutic Principles, 2nd Edition* (pp. 325–347). Cham: Springer. \[DOI:[10.1007/978-3-319-53298-1\_16][LTV17]]
|
||
- Mahfouda, S., Moore, J. K., Siafarikas, A., Hewitt, T., Ganti, U., Lin, A., & Zepf, F. D. (2019). Gender-affirming hormones and surgery in transgender children and adolescents. *The Lancet Diabetes & Endocrinology*, *7*(6), 484–498. \[DOI:[10.1016/s2213-8587(18)30305-x][M19]]
|
||
- Meriggiola, M. C., Bremner, W. J., Costantino, A., Di Cintio, G., & Flamigni, C. (1998). Low dose of cyproterone acetate and testosterone enanthate for contraception in men. *Human Reproduction*, *13*(5), 1225–1229. \[DOI:[10.1093/humrep/13.5.1225][M98]]
|
||
- Meriggiola, M. C., Bremner, W. J., Costantino, A., Bertaccini, A., Morselli-Labate, A. M., Huebler, D., Kaufmann, G., Oettel, M., & Flamigni, C. (2002). Twenty-One Day Administration of Dienogest Reversibly Suppresses Gonadotropins and Testosterone in Normal Men. *The Journal of Clinical Endocrinology & Metabolism*, *87*(5), 2107–2113. \[DOI:[10.1210/jcem.87.5.8514][M02a]]
|
||
- Meriggiola, M. C., Costantino, A., Bremner, W. J., & Morselli-Labate, A. M. (2002). Higher Testosterone Dose Impairs Sperm Suppression Induced by a Combined Androgen‐Progestin Regimen. *Journal of Andrology*, *23*(5), 684–690. \[DOI:[10.1002/j.1939-4640.2002.tb02311.x][M02b]]
|
||
- Meyer, G., Mayer, M., Mondorf, A., Flügel, A. K., Herrmann, E., & Bojunga, J. (2020). Safety and rapid efficacy of guideline-based gender-affirming hormone therapy: an analysis of 388 individuals diagnosed with gender dysphoria. *European Journal of Endocrinology*, *182*(2), 149–156. \[DOI:[10.1530/eje-19-0463][M20]] \[[PDF][M20-PDF]]
|
||
- Moltz, L., Römmler, A., Schwartz, U., & Hammerstein, J. (1978). Effects of Cyproterone Acetate (CPA) on Pituitary Gonadotrophin Release and on Androgen Secretion Before and After LH-RH Double Stimulation Tests in Men. *International Journal of Andrology*, *1*(Suppl 2b) \[*5th Annual Workshop on the Testis, Geilo, Norway, April 1978, Endocrine Approach to Male Contraception*], 713–719. \[DOI:[10.1111/j.1365-2605.1978.tb00518.x][M78b]]
|
||
- Moltz, L., Römmler, A., Schwartz, U., Post, K., & Hammerstein, J. (1978). Cyproterone acetate (CPA)—a potential male contraceptive: further studies on the interactions with endocrine parameters. *Journal of Steroid Biochemistry*, *9*(9), 865–865 (abstract no. 252). \[DOI:[10.1016/0022-4731(78)90952-4][M78a]]
|
||
- Moltz, L., Römmler, A., Post, K., Schwartz, U., & Hammerstein, J. (1980). Medium dose cyproterone acetate (CPA): Effects on hormone secretion and on spermatogenesis in men. *Contraception*, *21*(4), 393–413. \[DOI:[10.1016/s0010-7824(80)80017-5][M80]]
|
||
- Moltz, L., Koch, U., Schwartz, U., Rommler, A., & Hammerstein, J. (1982). Male fertility regulation with cyproterone acetate (CPA). *Contraceptive Delivery Systems*, *3*(3/4) \[*Retroproductive Health Care International Symposium, October 10-15 1982 Maui, Hawaii, USA, Expanded Abstracts*], 298–298 (abstract no. 293). \[[Google 学术][M82-GS]] \[[PDF][M82]]
|
||
- Moore, E., Wisniewski, A., & Dobs, A. (2003). Endocrine Treatment of Transsexual People: A Review of Treatment Regimens, Outcomes, and Adverse Effects. *The Journal of Clinical Endocrinology & Metabolism*, *88*(8), 3467–3473. \[DOI:[10.1210/jc.2002-021967][MWD03]]
|
||
- Nelson, J. B. (2012). Hormone Therapy for Prostate Cancer. In Wein, A. J., Kavoussi, L. R., Novick, A. C., Partin, A. W., & Peters, C. A. (Eds.). *Campbell-Walsh Urology, 10th Edition, Volume 2* (pp. 2920–2953). Philadelphia: Elsevier/Saunders. \[[Google 学术][N12-GS]] \[[Google 阅读][N12]]
|
||
- Nota, N. M., den Heijer, M., Gooren, L. J. (2019). Evaluation and Treatment of Gender-Dysphoric/Gender Incongruent Adults. \[Updated 2019 Jul 21]. In Feingold, K. R., Anawalt, B., Blackman, M. R., et al. (Eds.). *Endotext* \[Internet]. South Dartmouth, Massachusetts: MDText.com. \[[PubMed][NHG19]]
|
||
- Oliphant, J., Veale, J., Macdonald, J., Carroll, R., Johnson, R., Harte, M., Stephenson, C. & Bullock, J. (2018). *Guidelines for Gender Affirming Healthcare for Gender Diverse and Transgender Children, Young People and Adults in Aotearoa New Zealand*. Waikato: Transgender Health Research Lab/University of Waikato. \[[URL][O18]] \[[PDF][O18-PDF]]
|
||
- Ott, J., Aust, S., Promberger, R., Huber, J. C., & Kaufmann, U. (2011). Cross‐Sex Hormone Therapy Alters the Serum Lipid Profile: A Retrospective Cohort Study in 169 Transsexuals. *The Journal of Sexual Medicine*, *8*(8), 2361–2369. \[DOI:[10.1111/j.1743-6109.2011.02311.x][O11]]
|
||
- Petry, R., Mauss, J., Senge, T., & Rausch-Stroomann, J. (1970). Über den Einfluß von Cyproteronacetat, Norethisteronönanthat und Gestonoroncapronat auf die Hypophysen-Gonadenachse beim Mann. \[Influence of Cyproterone-acetate, Norethisterone-enanthate and Gestonorone-capronate on the Hypophyseal-Gonadal-Axis in the Male.] In Kracht, J. (Ed.). *Endokrinologie der Entwicklung und Reifung, 16. Symposion, Ulm, 26.-28. Februar 1970* (*Symposion der Deutschen Gesellschaft für Endokrinologie, Volume 16*) (pp. 428–430). Berlin: Springer. \[[Google 阅读][P70C-GB]] \[DOI:[10.1007/978-3-642-80591-2\_118][P70c]] \[[WorldCat][P70C-WC]] \[[PDF][P70C-PDF]]
|
||
- Petry, R., Rausch-Stroomann, J.-G., Berthold, K. Mauss, J., Ai, M., Senge, Th., & Vermeulen, A. (1970). Untersuchungen zum Wirkungsmechanismus der Antiandrogene Cyproteron und Cyproteronacetat beim Menschen (Gonadotropin-, Plasma-testosteron- und morphologische Keimdrüsenuntersuchungen). \[Investigations on the mechanism of action of the antiandrogens cyproterone and cyproterone acetate in humans (gonadotropin, plasma testosterone, and morphological gonad investigations).] In Schlegel, B. (Ed.). *Verhandlungen der Deutschen Gesellschaft für Innere Medizin: Sechsundsiebzigster Kongress Gehalten zu Wiesbaden vom 6. April – 9. April 1970* (*Verhandlungen der Deutschen Gesellschaft für Innere Medizin, Volume 76*) (pp. 873–876). München: Bergmann. \[[Google 学术][P70B-GS]] \[[Google 阅读][P70b]] \[DOI:[10.1007/978-3-642-85446-0][P70B-DOI]] \[[WorldCat][P70B-WC]] \[[PDF][P70B-PDF]]
|
||
- Petry, R., Rausch-Stroomann, J. G., Mauss, J., Senge, Th., Ai, M., & Berthold, K. (1970). Investigations on the mode of action of the antiandrogens cyproterone and cyproterone acetate in man. / Investigations on the mechanism of action of anti androgenic cyproterone and cyproterone acetate in humans (gonadotropin, plasma testosterone, and morphological generative gland investigations). *Medizinische Welt*, *29*, 1336–. \[[EurekaMag][P70a]] \[被 Koch et al. (1976) 所引用]
|
||
- Petry, R., Mauss, J., Rausch-Stroomann, J. G., & Vermeulen, A. (1972). Reversible inhibition of spermatogenesis in men. *Hormone and Metabolic Research*, *4*(5), 386–388. \[DOI:[10.1055/s-0028-1094040][P72]]
|
||
- Roy, S., Chatterjee, S., Prasad, M., Poddar, A., Pandey, D., Pandey, H., & Jadhav, Y. (1976). Effects of cyproterone acetate on reproductive functions in normal human males. *Contraception*, *14*(4), 403–423. \[DOI:[10.1016/s0010-7824(76)80055-8][R76]]
|
||
- Roy, S., & Chatterjee, S. (1979). Studies with cyproterone acetate for male contraception. In James, V. H. T., & Pasqualini, J. R. (Eds.). *Hormonal Steroids: Proceedings of the Fifth International Congress on Hormonal Steroids, New Delhi, India, October/November 1978* (pp. 675–680). Oxford: Pergamon Press. \[DOI:[10.1016/b978-0-08-023796-1.50099-2][RC79a]]
|
||
- Roy, S., & Chatterjee, S. (1979). The Role of Antiandrogenic Action in Cyproterone Acetate-Induced Morphologic and Biochemical Changes in Human Semen. *Fertility and Sterility*, *32*(1), 93–95. \[DOI:[10.1016/s0015-0282(16)44122-1][RC79b]]
|
||
- Saborowski, K.-J. (1987). Konservative Therapie mit Cyproteronacetat und Estradiolundecylat beim Fortgeschrittenen Prostatacarcinom: Eine 5-Jahres-Studie. \[Conservative Therapy with Cyproterone Acetate and Estradiol Undecylate in Advanced Prostate Cancer: A 5-Year Study.] (Doctoral dissertation, Ruhr-University Bochum.) \[共 58 页] \[[Google 学术][S87-GS]] \[[Google 阅读][S87-GB]] \[[WorldCat][S87]] \[[PDF][S87-PDF]] \[[英译本][S87-ENG]]
|
||
- Scharff, M., Wiepjes, C. M., Klaver, M., Schreiner, T., T’Sjoen, G., & den Heijer, M. (2019). Change in grip strength in trans people and its association with lean body mass and bone density. *Endocrine Connections*, *8*(7), 1020–1028. \[DOI:[10.1530/ec-19-0196][S19]]
|
||
- Schröder, F. H., & Radlmaier, A. (2002). Steroidal Antiandrogens. In Jordan, C. V., & Furr, B. J. A. (Eds.). *Hormone Therapy in Breast and Prostate Cancer* (pp. 325–346). Totowa, New Jersey: Humana Press. \[DOI:[10.1007/978-1-59259-152-7\_15][SR02]]
|
||
- Slagter, M. H., Gooren, L. J., de Ronde, W., Soosaipillai, A., Scorilas, A., Giltay, E. J., Paliouras, M., & Diamandis, E. P. (2006). Serum and Urine Tissue Kallikrein Concentrations in Male-to-Female Transsexuals Treated with Antiandrogens and Estrogens. *Clinical Chemistry*, *52*(7), 1356–1365. \[DOI:[10.1373/clinchem.2006.068932][S06]]
|
||
- Sofer, Y., Yaish, I., Yaron, M., Bach, M. Y., Stern, N., & Greenman, Y. (2020). Differential Endocrine and Metabolic Effects of Testosterone Suppressive Agents in Transgender Women. *Endocrine Practice*, *26*(8), 883–890. \[DOI:[10.4158/ep-2020-0032][S20]] \[[PDF][S20-PDF]]
|
||
- T’Sjoen, G. G., Beguin, Y., Feyen, E., Rubens, R., Kaufman, J., & Gooren, L. (2005). Influence of exogenous oestrogen or (anti-) androgen administration on soluble transferrin receptor in human plasma. *Journal of Endocrinology*, *186*(1), 61–67. \[DOI:[10.1677/joe.1.06112][TS05]]
|
||
- T’Sjoen, G., Weyers, S., Taes, Y., Lapauw, B., Toye, K., Goemaere, S., & Kaufman, J. (2009). Prevalence of Low Bone Mass in Relation to Estrogen Treatment and Body Composition in Male-to-Female Transsexual Persons. *Journal of Clinical Densitometry*, *12*(3), 306–313. \[DOI:[10.1016/j.jocd.2008.11.002][TS09]]
|
||
- T’Sjoen, G., Arcelus, J., De Vries, A. L., Fisher, A. D., Nieder, T. O., Özer, M., & Motmans, J. (2020). European Society for Sexual Medicine Position Statement “Assessment and Hormonal Management in Adolescent and Adult Trans People, with Attention for Sexual Function and Satisfaction”. *The Journal of Sexual Medicine*, *17*(4), 570–584. \[DOI:[10.1016/j.jsxm.2020.01.012][TS20]]
|
||
- Tack, L. J., Heyse, R., Craen, M., Dhondt, K., Bossche, H. V., Laridaen, J., & Cools, M. (2017). Consecutive Cyproterone Acetate and Estradiol Treatment in Late-Pubertal Transgender Female Adolescents. *The Journal of Sexual Medicine*, *14*(5), 747–757. \[DOI:[10.1016/j.jsxm.2017.03.251][T17]]
|
||
- Toorians, A. W., Thomassen, M. C., Zweegman, S., Magdeleyns, E. J., Tans, G., Gooren, L. J., & Rosing, J. (2003). Venous Thrombosis and Changes of Hemostatic Variables during Cross-Sex Hormone Treatment in Transsexual People. *The Journal of Clinical Endocrinology & Metabolism*, *88*(12), 5723–5729. \[DOI:[10.1210/jc.2003-030520][T03]]
|
||
- Torre, B. l., Norén, S., Hedman, M., & Diczfalusy, E. (1979). Effect of cyproterone acetate (CPA) on gonadal and adrenal function in men. *Contraception*, *20*(4), 377–396. \[DOI:[10.1016/s0010-7824(79)80048-7][T79]]
|
||
- Van Caenegem, E., Wierckx, K., Taes, Y., Schreiner, T., Vandewalle, S., Toye, K., Kaufman, J., & T’Sjoen, G. (2015). Preservation of volumetric bone density and geometry in trans women during cross-sex hormonal therapy: a prospective observational study. *Osteoporosis International*, *26*(1), 35–47. \[DOI:[10.1007/s00198-014-2805-3][VC15]]
|
||
- van Dijk, D., Dekker, M. J., Conemans, E. B., Wiepjes, C. M., de Goeij, E. G., Overbeek, K. A., Fisher, A. D., den Heijer, M., & T’Sjoen, G. (2019). Explorative Prospective Evaluation of Short-Term Subjective Effects of Hormonal Treatment in Trans People—Results from the European Network for the Investigation of Gender Incongruence. *The Journal of Sexual Medicine*, *16*(8), 1297–1309. \[DOI:[10.1016/j.jsxm.2019.05.009][VD19]]
|
||
- van Velzen, D. M., Paldino, A., Klaver, M., Nota, N. M., Defreyne, J., Hovingh, G. K., Thijs, A., Simsek, S., T’Sjoen, G., & den Heijer, M. (2019). Cardiometabolic Effects of Testosterone in Transmen and Estrogen Plus Cyproterone Acetate in Transwomen. *The Journal of Clinical Endocrinology & Metabolism*, *104*(6), 1937–1947. \[DOI:[10.1210/jc.2018-02138][VV19]]
|
||
- Venner, P. M., Klotz, P. G., Klotz, L. H., Stewart, D. J., Davis, I. R., Orovan, W. L., & Ramsey, E. W. (1988). Megestrol acetate plus minidose diethylstilbestrol in the treatment of carcinoma of the prostate. *Seminars in Oncology*, *15*(2 Suppl 1), 62–67. \[[Google 学术][V88-GS]] \[[PubMed][V88]]
|
||
- Vereecke, G. (2019). *Characterisation of testicular function and spermatogenesis in transgender women.* (Master’s thesis, Ghent University.) \[[PDF][V19]]
|
||
- Vereecke, G., Defreyne, J., Van Saen, D., Collet, S., Van Dorpe, J., T’Sjoen, G., & Goossens, E. (2021). Characterisation of testicular function and spermatogenesis in transgender women. *Human Reproduction*, *36*(1), 5–15. \[DOI:[10.1093/humrep/deaa254][V21]]
|
||
- Vita, R., Settineri, S., Liotta, M., Benvenga, S., & Trimarchi, F. (2018). Changes in hormonal and metabolic parameters in transgender subjects on cross-sex hormone therapy: A cohort study. *Maturitas*, *107*, 92–96. \[DOI:[10.1016/j.maturitas.2017.10.012][V18]]
|
||
- Vlot, M. C., Wiepjes, C. M., Jongh, R. T., T’Sjoen, G., Heijboer, A. C., & den Heijer, M. (2019). Gender‐Affirming Hormone Treatment Decreases Bone Turnover in Transwomen and Older Transmen. *Journal of Bone and Mineral Research*, *34*(10), 1862–1872. \[DOI:[10.1002/jbmr.3762][VLOT19]]
|
||
- Wang, C., & Yeung, K. (1980). Use of low-dosage oral cyproterone acetate as a male contraceptive. *Contraception*, *21*(3), 245–272. \[DOI:[10.1016/0010-7824(80)90005-0][WY80]]
|
||
- Wiepjes, C. M., Vlot, M. C., Klaver, M., Nota, N. M., de Blok, C. J., de Jongh, R. T., Lips, P., Heijboer, A. C., Fisher, A. D., Schreiner, T., T’Sjoen, G., & den Heijer, M. (2017). Bone Mineral Density Increases in Trans Persons After 1 Year of Hormonal Treatment: A Multicenter Prospective Observational Study. *Journal of Bone and Mineral Research*, *32*(6), 1252–1260. \[DOI:[10.1002/jbmr.3102][W17]]
|
||
- Wiepjes, C. M., Vlot, M. C., de Blok, C. J., Nota, N. M., de Jongh, R. T., & den Heijer, M. (2019). Bone geometry and trabecular bone score in transgender people before and after short- and long-term hormonal treatment. *Bone*, *127*, 280–286. \[DOI:[10.1016/j.bone.2019.06.029][W19]]
|
||
- Wierckx, K., Mueller, S., Weyers, S., Van Caenegem, E., Roef, G., Heylens, G., & T’Sjoen, G. (2012). Long‐Term Evaluation of Cross‐Sex Hormone Treatment in Transsexual Persons. *The Journal of Sexual Medicine*, *9*(10), 2641–2651. \[DOI:[10.1111/j.1743-6109.2012.02876.x][W12]]
|
||
- Wierckx, K., Van Caenegem, E., Schreiner, T., Haraldsen, I., Fisher, A., Toye, K., Kaufman, J. M., & T’Sjoen, G. (2014). Cross‐Sex Hormone Therapy in Trans Persons Is Safe and Effective at Short‐Time Follow‐Up: Results from the European Network for the Investigation of Gender Incongruence. *The Journal of Sexual Medicine*, *11*(8), 1999–2011. \[DOI:[10.1111/jsm.12571][W14]]
|
||
- Winkler-Crepaz, K., Müller, A., Böttcher, B., & Wildt, L. (2017). Hormonbehandlung bei Transgenderpatienten. \[Hormone treatment of transgender patients.] *Gynäkologische Endokrinologie*, *15*(1), 39–42. \[DOI:[10.1007/s10304-016-0116-9][WC17]]
|
||
- Winters, S. J., Wang, C., & Fortigel Study Group. (2013). LH and Non-SHBG Testosterone and Estradiol Levels During Testosterone Replacement of Hypogonadal Men: Further Evidence That Steroid Negative Feedback Increases as Men Grow Older. *Journal of Andrology*, *31*(3), 281–287. \[DOI:[10.2164/jandrol.109.009035][WWF13]]
|
||
- Zitzmann, M., Rohayem, J., Raidt, J., Kliesch, S., Kumar, N., Sitruk-Ware, R., & Nieschlag, E. (2017). Impact of various progestins with or without transdermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: a randomized clinical trial. *Andrology*, *5*(3), 516–526. \[DOI:[10.1111/andr.12328][Z17]]
|
||
- Zubiaurre-Elorza, L., Junque, C., Gómez-Gil, E., & Guillamon, A. (2014). Effects of Cross-Sex Hormone Treatment on Cortical Thickness in Transsexual Individuals. *The Journal of Sexual Medicine*, *11*(5), 1248–1261. \[DOI:[10.1111/jsm.12491][ZE14]]
|
||
|
||
|
||
---
|
||
|
||
## 译文修订历史 {#revise-history}
|
||
|
||
```csv
|
||
时间,备注
|
||
2022 年 3 月 2 日,首次翻译。
|
||
2022 年 10 月 21 日,第一次修订,增补“后记四”“后记五”内容,整理外链。
|
||
2023 年 3 月 24 日,第二次修订,增补“参考文献”,补充遗漏或有变动的叙述,补全并更新外链。
|
||
2023 年 3 月 29 日,更新诸后记标题。
|
||
2023 年 4 月 4 日,第三次修订,增补“表一”和诸表格标题,更新个别叙述,添加相关文献。
|
||
2023 年 6 月 29 日,更正“己烯雌酚”译名。
|
||
```
|
||
|
||
<!-- 外源图片及表格 -->
|
||
|
||
[graph1]: https://commons.wikimedia.org/wiki/File:Testosterone_levels_with_5_or_10_mg_per_day_oral_cyproterone_acetate_in_men.png
|
||
[graph2]: https://commons.wikimedia.org/wiki/File:Androgen_levels_with_10_or_20_mg_per_day_oral_cyproterone_acetate_in_men.png
|
||
[graph3]: https://en.wikipedia.org/wiki/Template:Antigonadotropic_effects_of_estradiol
|
||
[graph4]: https://commons.wikimedia.org/wiki/File:Hormone_levels_with_low-dose_estradiol_gel_and_oral_cyproterone_acetate_in_transgender_women.png
|
||
[graph5]: https://commons.wikimedia.org/wiki/File:Testosterone_levels_with_estradiol_alone,_estradiol_plus_spironolactone,_and_estradiol_plus_cyproterone_acetate_in_transfeminine_people.png
|
||
[graph6]: https://commons.wikimedia.org/wiki/File:Cyproterone_acetate_levels_with_100_mg_oral_cyproterone_acetate_per_day_in_women.png
|
||
[graph7]: https://commons.wikimedia.org/wiki/File:Testosterone_and_luteinizing_hormone_levels_with_100_mg_per_day_oral_cyproterone_acetate_in_men.png
|
||
[graph8]: https://commons.wikimedia.org/wiki/File:Testosterone_levels_with_300_mg_per_week_cyproterone_acetate_and_100_mg_per_month_estradiol_undecylate_by_intramuscular_injection.png
|
||
[graph9]: https://commons.wikimedia.org/wiki/File:Testosterone_levels_in_men_during_treatment_with_various_progestins_alone_then_combined_with_testosterone_followed_by_discontinuation.png#%7B%7Bint%3Afiledesc%7D%7D
|
||
[graph10]: https://commons.wikimedia.org/wiki/File:Testosterone_levels_with_different_doses_of_dienogest_and_cyproterone_acetate_in_men.png
|
||
[graph11]: https://en.wikipedia.org/wiki/Template:Testosterone_levels_with_cyproterone_acetate
|
||
|
||
[table1]: https://en.wikipedia.org/wiki/Template:Oral_potencies_of_progestogens
|
||
[table2]: https://en.wikipedia.org/wiki/Template:Published_case_reports_of_cyproterone_acetate-associated_meningioma
|
||
[table3]: https://en.wikipedia.org/wiki/Template:Published_case_reports_of_cyproterone_acetate-associated_prolactinoma
|
||
[table4]: https://en.wikipedia.org/wiki/Template:Published_case_reports_of_cyproterone_acetate-associated_liver_toxicity
|
||
|
||
[pic1]: https://imgur.com/a/SAr46aj
|
||
|
||
<!-- 维基百科条目 -->
|
||
|
||
[wiki1]: https://en.wikipedia.org/wiki/Cyproterone_acetate
|
||
[wiki1-mu]: https://en.wikipedia.org/wiki/Cyproterone_acetate#Medical_uses
|
||
[wiki1-af]: https://en.wikipedia.org/wiki/Cyproterone_acetate#Available_forms
|
||
[wiki2-pd]: https://en.wikipedia.org/wiki/Pharmacology_of_cyproterone_acetate#Pharmacodynamics
|
||
[wiki2-m]: https://en.wikipedia.org/wiki/Pharmacology_of_cyproterone_acetate#Metabolism
|
||
[wiki3]: https://en.wikipedia.org/wiki/Chlormadinone_acetate
|
||
[wiki4]: https://en.wikipedia.org/wiki/Cyproterone
|
||
[wiki5]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate
|
||
[wiki5-hpl]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate#High_prolactin_levels
|
||
[wiki5-bt]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate#Brain_tumors
|
||
[wiki5-bc]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate#Blood_clots
|
||
[wiki5-ce]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate#Cardiovascular_effects
|
||
[wiki5-lt]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate#Liver_toxicity
|
||
[wiki5-lhl]: https://en.wikipedia.org/wiki/Side_effects_of_cyproterone_acetate#Low_hormone_levels
|
||
[wiki6]: https://en.wikipedia.org/wiki/Desogestrel
|
||
[wiki6-ae]: https://en.wikipedia.org/wiki/Desogestrel#Antigonadotropic_effects
|
||
[wiki7]: https://en.wikipedia.org/wiki/Dienogest
|
||
[wiki7-ae]: https://en.wikipedia.org/wiki/Dienogest#Antigonadotropic_effects
|
||
[wiki8]: https://en.wikipedia.org/wiki/Medroxyprogesterone_acetate
|
||
[wiki8-aae]: https://en.wikipedia.org/wiki/Medroxyprogesterone_acetate#Antigonadotropic_and_anticorticotropic_effects
|
||
[wiki9]: https://en.wikipedia.org/wiki/Norethisterone_acetate
|
||
[wiki10]: https://en.wikipedia.org/wiki/Levonorgestrel
|
||
[wiki11]: https://en.wikipedia.org/wiki/Male_contraceptive
|
||
[wiki12]: https://en.wikipedia.org/wiki/Megestrol_acetate
|
||
[wiki12-aae]: https://en.wikipedia.org/wiki/Megestrol_acetate#Antigonadotropic_and_anticorticotropic_effects
|
||
[wiki13-eoshl]: https://en.wikipedia.org/wiki/Pharmacodynamics_of_estradiol#Effects_on_sex-hormone_levels
|
||
[wiki14]: https://en.wikipedia.org/wiki/Diethylstilbestrol
|
||
[wiki15]: https://en.wikipedia.org/wiki/Pharmacokinetics_of_estradiol
|
||
[wiki16]: https://en.wikipedia.org/wiki/Pharmacology_of_bicalutamide#Influences_on_hormone_levels
|
||
[wiki17]: https://en.wikipedia.org/wiki/Dose-ranging_study
|
||
[wiki18]: https://en.wikipedia.org/wiki/European_Network_for_the_Investigation_of_Gender_Incongruence
|
||
[wiki19]: https://nl.wikipedia.org/wiki/Guy_T%27Sjoen
|
||
[wiki20]: https://en.wikipedia.org/wiki/Liver_failure
|
||
[wiki21]: https://en.wikipedia.org/wiki/Hirsutism
|
||
[wiki22]: https://en.wikipedia.org/wiki/Acute_liver_failure
|
||
[wiki23]: https://en.wikipedia.org/wiki/Liver_toxicity
|
||
[wiki24]: https://zh.wikipedia.org/wiki/%E4%B8%96%E7%95%8C%E8%B7%A8%E6%80%A7%E5%88%AB%E4%BA%BA%E5%A3%AB%E5%81%A5%E5%BA%B7%E4%B8%93%E4%B8%9A%E5%8D%8F%E4%BC%9A
|
||
[wiki25]: https://en.wikipedia.org/wiki/Standards_of_Care_for_the_Health_of_Transgender_and_Gender_Diverse_People
|
||
[wiki26]: https://en.wikipedia.org/wiki/Adrenal_androgen
|
||
[wiki27]: https://en.wikipedia.org/wiki/Dehydroepiandrosterone
|
||
[wiki28]: https://en.wikipedia.org/wiki/Dehydroepiandrosterone_sulfate
|
||
[wiki29]: https://en.wikipedia.org/wiki/Androstenedione
|
||
[wiki30]: https://en.wikipedia.org/wiki/Liquid_chromatography%E2%80%93mass_spectrometry
|
||
[wiki31]: https://en.wikipedia.org/wiki/Immunoassay
|
||
[wiki32]: https://en.wikipedia.org/wiki/Ovulation-inhibiting_dosage
|
||
[wiki33]: https://en.wikipedia.org/wiki/Endometrial_transformation_dosage
|
||
|
||
<!-- 参考文献链接 -->
|
||
|
||
[E11]: https://doi.org/10.1016/j.contraception.2011.04.009
|
||
[H75]: https://doi.org/10.1016/0022-4731(75)90311-8
|
||
[H90]: https://doi.org/10.1007/978-3-642-74612-3_35
|
||
[H79]: https://books.google.com/books?id=waMTAQAAMAAJ
|
||
[AW20-CM]: https://transfemscience.org/articles/cpa-meningioma/
|
||
[MWD03]: https://doi.org/10.1210/jc.2002-021967
|
||
[H17]: https://doi.org/10.1210/jc.2017-01658
|
||
[H09]: https://doi.org/10.1210/jc.2009-0345
|
||
[WY80]: https://doi.org/10.1016/0010-7824(80)90005-0
|
||
[K76]: https://doi.org/10.1016/0010-7824(76)90081-0
|
||
[K75]: https://doi.org/10.1007/BF00669258
|
||
[M02a]: https://doi.org/10.1210/jcem.87.5.8514
|
||
[Z17]: https://doi.org/10.1111/andr.12328
|
||
[P72]: https://doi.org/10.1055/s-0028-1094040
|
||
[P70a]: https://eurekamag.com/research/026/853/026853674.php
|
||
[P70b]: https://books.google.com/books?id=7ICiBgAAQBAJ&pg=PA873
|
||
[P70c]: https://doi.org/10.1007/978-3-642-80591-2_118
|
||
[R76]: https://doi.org/10.1016/S0010-7824%2876%2980055-8
|
||
[M80]: https://doi.org/10.1016/S0010-7824%2880%2980017-5
|
||
[M78a]: https://doi.org/10.1016/0022-4731%2878%2990952-4
|
||
[M78b]: https://doi.org/10.1111/j.1365-2605.1978.tb00518.x
|
||
[RC79a]: https://doi.org/10.1016/B978-0-08-023796-1.50099-2
|
||
[RC79b]: https://doi.org/10.1016/S0015-0282%2816%2944122-1
|
||
[T79]: https://doi.org/10.1016/S0010-7824%2879%2980048-7
|
||
[F79]: https://doi.org/10.1530/acta.0.0910545
|
||
[DP80]: https://doi.org/10.1530/acta.0.0940280
|
||
[F80]: https://doi.org/10.1530/acta.0.0940430
|
||
[F83]: https://doi.org/10.1530/acta.0.104s009
|
||
[FC81]: https://doi.org/10.1111/j.1439-0272.1981.tb00067.x
|
||
[M82]: https://files.transfemscience.org/pdfs/Moltz%20et%20al.%20%281982%29%20-%20Male%20Fertility%20Regulation%20with%20Cyproterone%20Acetate%20%28CPA%29.pdf#page=4
|
||
[T03]: https://doi.org/10.1210/jc.2003-030520
|
||
[G04]: https://doi.org/10.1093/ajcn/80.5.1167
|
||
[TS05]: https://doi.org/10.1677/joe.1.06112
|
||
[T17]: https://doi.org/10.1016/j.jsxm.2017.03.251
|
||
[GBN74]: https://doi.org/10.1007/978-3-642-80859-3_7
|
||
[J80]: https://doi.org/10.1111/j.1464-410X.1980.tb02961.x
|
||
[KHN84]: https://doi.org/10.1210/jcem-59-5-963
|
||
[SR02]: https://doi.org/10.1007/978-1-59259-152-7_15
|
||
[N12]: https://books.google.com/books?id=fu3BBwAAQBAJ&pg=PA2938
|
||
[LTV17]: https://doi.org/10.1007/978-3-319-53298-1_16
|
||
[WWF13]: https://doi.org/10.2164/jandrol.109.009035
|
||
[GB91]: https://doi.org/10.1016/S0094-0143(21)01398-7
|
||
[S87]: https://www.worldcat.org/title/konservative-therapie-mit-cyproteronacetat-und-estradiolundecylat-beim-fortgeschrittenen-prostatacarcinom-eine-5-jahres-studie/oclc/917571781
|
||
[JTS82]: https://scholar.google.com/scholar?cluster=8992751753331497790
|
||
[FINK79]: https://doi.org/10.1146/annurev.ph.41.030179.003035
|
||
[GA83]: https://files.transfemscience.org/pdfs/Geller%20&%20Albert%20(1983)%20-%20Comparison%20of%20Various%20Hormonal%20Therapies%20for%20Prostatic%20Carcinoma.pdf
|
||
[B18]: https://doi.org/10.1080/17512433.2018.1536544
|
||
[GG00]: https://doi.org/10.1210/jcem.85.8.6710
|
||
[G00]: https://doi.org/10.1161/01.ATV.20.5.1396
|
||
[G03]: https://doi.org/10.1016/s0021-9150(03)00090-x
|
||
[S06]: https://doi.org/10.1373/clinchem.2006.068932
|
||
[TS09]: https://doi.org/10.1016/j.jocd.2008.11.002
|
||
[O11]: https://doi.org/10.1111/j.1743-6109.2011.02311.x
|
||
[W12]: https://doi.org/10.1111/j.1743-6109.2012.02876.x
|
||
[W14]: https://doi.org/10.1111/jsm.12571
|
||
[ZE14]: https://doi.org/10.1111/jsm.12491
|
||
[F15]: https://doi.org/10.1016/j.euroneuro.2014.11.019
|
||
[VC15]: https://doi.org/10.1007/s00198-014-2805-3
|
||
[G16]: https://doi.org/10.1111/cen.13050
|
||
[B17]: https://doi.org/10.1002/lary.26716
|
||
[FHL17]: https://doi.org/10.1080/15532739.2017.1290566
|
||
[K17]: https://doi.org/10.1016/j.neuroimage.2017.02.027
|
||
[W17]: https://doi.org/10.1002/jbmr.3102
|
||
[DB17]: https://doi.org/10.1210/jc.2017-01927
|
||
[D18]: https://doi.org/10.1111/andr.12485
|
||
[V18]: https://doi.org/10.1016/j.maturitas.2017.10.012
|
||
[A19]: https://doi.org/10.1530/EC-19-0272
|
||
[C19]: https://doi.org/10.1210/jc.2018-02602
|
||
[S19]: https://doi.org/10.1530/EC-19-0196
|
||
[VD19]: https://doi.org/10.1016/j.jsxm.2019.05.009
|
||
[VV19]: https://doi.org/10.1210/jc.2018-02138
|
||
[V19]: https://lib.ugent.be/fulltxt/RUG01/002/783/592/RUG01-002783592_2019_0001_AC.pdf
|
||
[VLOT19]: https://doi.org/10.1002/jbmr.3762
|
||
[W19]: https://doi.org/10.1016/j.bone.2019.06.029
|
||
[KKL20]: https://doi.org/10.1093/chemse/bjz069
|
||
[M20]: https://doi.org/10.1530/eje-19-0463
|
||
[G20]: https://doi.org/10.1530/eje-20-0370
|
||
[S20]: https://doi.org/10.4158/ep-2020-0032
|
||
[V21]: https://doi.org/10.1093/humrep/deaa254
|
||
[G81a]: https://doi.org/10.1210/jcem-52-3-576
|
||
[G81b]: https://www.ncbi.nlm.nih.gov/pubmed/6782738
|
||
[G88]: https://doi.org/10.1016/s0022-5347(17)42073-8
|
||
[J88]: https://doi.org/10.1016/0090-4295(88)90726-1
|
||
[GEL88]: https://doi.org/10.1016/0090-4295(88)90402-5
|
||
[V88]: https://www.ncbi.nlm.nih.gov/pubmed/3285485
|
||
[G91-PDF]: https://files.transfemscience.org/pdfs/Geller%20(1991)%20-%20Megestrol%20Acetate%20Plus%20Low-Dose%20Estrogen%20in%20the%20Management%20of%20Advanced%20Prostatic%20Carcinoma.pdf
|
||
[B93]: https://doi.org/10.1002/1097-0142(19930901)72:5%3C1685::AID-CNCR2820720532%3E3.0.CO;2-3
|
||
[G96]: https://doi.org/10.1016/S0090-4295(96)00048-9
|
||
[JBB89]: https://doi.org/10.1111/j.1439-0272.1989.tb02447.x
|
||
[AW20-EED]: https://transfemscience.org/articles/e2-equivalent-doses/
|
||
[M98]: https://doi.org/10.1093/humrep/13.5.1225
|
||
[M02b]: https://doi.org/10.1002/j.1939-4640.2002.tb02311.x
|
||
[EZ20]: https://doi.org/10.1210/jendso/bvaa046.1412
|
||
[JKF19]: https://doi.org/10.1210/jc.2018-02253
|
||
[L09]: http://doi.org/10.1055/s-0028-1090190
|
||
[HW19]: https://books.google.com/books?id=-sCZDwAAQBAJ&pg=PA122
|
||
[WC17]: https://doi.org/10.1007/s10304-016-0116-9
|
||
[M19]: https://doi.org/10.1016/s2213-8587(18)30305-x
|
||
[O18]: https://hdl.handle.net/10289/12160
|
||
[AKS19]: http://doi.org/10.1055/a-0801-3319
|
||
[B19]: https://www.rainbowhealthontario.ca/resources/4th-edition-sherbournes-guidelines-for-gender-affirming-primary-care-with-trans-and-non-binary-patients/
|
||
[F19]: https://doi.org/10.1016/j.psyneuen.2019.02.023
|
||
[J19]: https://doi.org/10.1007/s41969-019-00080-x
|
||
[NHG19]: https://www.ncbi.nlm.nih.gov/books/NBK544426/
|
||
[A20]: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379339
|
||
[TS20]: https://doi.org/10.1016/j.jsxm.2020.01.012
|
||
[AG93]: https://doi.org/10.1300/J056v05n04_03
|
||
[GGB08]: https://doi.org/10.1210/jc.2007-1809
|
||
|
||
[AW19-NTO]: https://transfemscience.org/articles/nonbinary-transfem-overview/
|
||
[GLC]: https://researchers.uq.edu.au/research-project/38285
|
||
[K21]: https://doi.org/10.1210/clinem/dgab427
|
||
[G21]: https://doi.org/10.1530/EJE-21-0059
|
||
[EZ21]: https://doi.org/10.1016/j.jsxm.2021.04.008
|
||
[L20]: https://doi.org/10.1210/clinem/dgaa262
|
||
[KUMAR21]: https://doi.org/10.1016/j.jceh.2021.01.003
|
||
[C22]: https://doi.org/10.1080/26895269.2022.2100644
|
||
[C23]: https://doi.org/10.1210/clinem/dgac576
|
||
|
||
[AG93-GS]: https://scholar.google.com/scholar?cluster=3926911364428297742
|
||
[AG93-GB]: https://books.google.com/books?id=fny-DwAAQBAJ&pg=PT38
|
||
[B19-PDF]: https://www.rainbowhealthontario.ca/wp-content/uploads/woocommerce_uploads/2019/12/Guidelines-FINAL-4TH-EDITION-n7ozcr.pdf
|
||
[C22-URL]: https://www.wpath.org/publications/soc
|
||
[C22-PDF]: https://www.tandfonline.com/doi/pdf/10.1080/26895269.2022.2100644
|
||
[G81b-GS]: https://scholar.google.com/scholar?cluster=3393124245397979807
|
||
[GA83-GS]: https://scholar.google.com/scholar?cluster=1336019387433653117
|
||
[GA83-PM]: https://pubmed.ncbi.nlm.nih.gov/6199846/
|
||
[G91]: https://doi.org/10.1016/S0094-0143(21)01395-1
|
||
[G00]: https://doi.org/10.1161/01.atv.20.5.1396
|
||
[GB91-PDF]: https://files.transfemscience.org/pdfs/Goldenberg%20&%20Bruchovsy%20(1991)%20-%20Use%20of%20Cyproterone%20Acetate%20in%20Prostate%20Cancer.pdf
|
||
[H79-GS]: https://scholar.google.com/scholar?cluster=18400175768465948023
|
||
[H79-GB]: https://books.google.com/books?id=waMTAQAAMAAJ
|
||
[H17-PDF]: https://files.transfemscience.org/pdfs/Hembree%20et%20al.%20(2017)%20-%20Endocrine%20Treatment%20of%20Gender-Dysphoric_Gender-Incongruent%20Persons_%20An%20Endocrine%20Society_%20Clinical%20Practice%20Guideline.pdf
|
||
[J80]: https://doi.org/10.1111/j.1464-410x.1980.tb02961.x
|
||
[JTS82-GS]: https://scholar.google.com/scholar?cluster=8992751753331497790
|
||
[M20-PDF]: https://files.transfemscience.org/pdfs/Meyer%20et%20al.%20(2020)%20-%20Safety%20and%20Rapid%20Efficacy%20of%20Guideline-Based%20Gender-Affirming%20Hormone%20Therapy_%20An%20Analysis%20of%20388%20Individuals%20Diagnosed%20with%20Gender%20Dysphoria.pdf
|
||
[M82-GS]: https://scholar.google.com/scholar?cluster=9581511158084858663
|
||
[N12-GS]: https://scholar.google.com/scholar?cluster=17139352108227037971
|
||
[O18-PDF]: https://researchcommons.waikato.ac.nz/bitstream/handle/10289/12160/Guidelines%20for%20Gender%20Affirming%20Health%20low%20res.pdf
|
||
[P70C-GB]: https://books.google.com/books?id=bAihBgAAQBAJ&%3Bpg=PA428
|
||
[P70C-WC]: https://worldcat.org/title/419096
|
||
[P70C-PDF]: https://files.transfemscience.org/pdfs/Petry%20et%20al.%20%281970%29%20-%20%C3%9Cber%20den%20Einflu%C3%9F%20von%20Cyproteronacetat,%20Norethisteron%C3%B6nanthat%20und%20Gestonoroncapronat%20auf%20die%20Hypophysen-Gonadenachse%20beim%20Mann.pdf
|
||
[P70B-GS]: https://scholar.google.com/scholar?cluster=16507195795753926943
|
||
[P70B-DOI]: https://doi.org/10.1007/978-3-642-85446-0
|
||
[P70B-WC]: https://worldcat.org/title/35514355
|
||
[P70B-PDF]: https://files.transfemscience.org/pdfs/Petry%20et%20al.%20%281970%29%20-%20Untersuchungen%20zum%20Wirkungsmechanismus%20der%20Antiandrogene%20Cyproteron%20und%20Cyproteronacetat%20beim%20Menschen%20%28Gonadotropin-,%20Plasma-testosteron-%20und%20morphologische%20Keimdr%C3%BCsenuntersuchungen%29.pdf
|
||
[S87-GS]: https://scholar.google.com/scholar?as_sdt=1%2C5&q=author%3A%22KJ+saborowski%22&hl=en
|
||
[S87-GB]: https://books.google.com/books?id=9JeKGwAACAAJ
|
||
[S87-PDF]: https://files.transfemscience.org/pdfs/Saborowski%20(1987)%20-%20Konservative%20Therapie%20mit%20Cyproteronacetat%20und%20Estradiolundecylat%20beim%20Fortgeschrittenen%20Prostatacarcinom_%20Eine%205-Jahres-Studie.pdf
|
||
[S87-ENG]: https://docs.google.com/document/d/1s_Z4BXEd3chb4jG6eOSHoSbAwRCynhV3LtKKD_ZI3H8/view
|
||
[S20-PDF]: https://files.transfemscience.org/pdfs/Sofer%20et%20al.%20(2020)%20-%20Differential%20Endocrine%20and%20Metabolic%20Effects%20of%20Testosterone%20Suppressive%20Agents%20in%20Transgender%20Women.pdf
|
||
[V88-GS]: https://scholar.google.com/scholar?cluster=2098198133343723877 |